Introduction
Gyrodactylus von Nordmann, 1832 species have been reported as parasites of both marine and freshwater fishes globally (Harris et al. Reference Harris, Shinn, Cable and Bakke2004). Forty-one species have been described from Africa (Christison et al. Reference Christison, Vaugham, Shinn and Hansen2021; Dos Santos et al. Reference Dos Santos, Maina and Avenant-Oldewage2019b; Van As & Basson Reference Van As and Basson1984; Řehulková et al. Reference Řehulková, Seifertová, Přikrylová, Francová, Scholz, Vanhove, Smit, Jayasundera and Gelnar2018; Truter et al. Reference Truter, Smit, Malherbe and Přikrylová2022), with nine descriptions or records from South African freshwater and marine fishes (Table 1). Only three of these species parasitize cyprinids, Gyrodactylus kherulensis Ergens, 1974, Gyrodactylus kobayashii Hukuda, 1940 and Gyrodactylus paludinosus (Truter et al. Reference Truter, Smit, Malherbe and Přikrylová2022). The last taxon described was from South Africa, whereas G. kherulensis and G. kobayashii are suspected to be co-introduced with their ornamental and aquaculture fish host species, Cyprinus carpio Linnaeus, 1758 and Carassius auratus (Linnaeus, 1758) respectively (Smit et al. Reference Smit, Malherbe and Hadfield2017).
Table 1. List of Gyrodactylus species described or reported from South African marine and freshwater fishes. +Description, ++Distribution record, *Freshwater, **Marine.

Gyrodactylus sprostonae Ling, 1962, was first described from the gills of C. auratus and C. carpio in the middle and lower reaches of the Liaohe River, China (Ling Reference Ling1962). Since then, it has been reported in other parts of Asia (e.g., Russia and China (Bykhovskaya-Pavlovskaya et al. Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964)), Japan (Ogawa & Egusa Reference Ogawa and Egusa1978), Iraq (Abdullah Reference Abdullah2013; Mhaisen & Abdul-Ameer Reference Mhaisen and Abdul-Ameer2013), Iran (Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019)), North America (e.g., Mendoza-Garfias et al. Reference Mendoza-Garfias, García-Prieto and Pérez-Ponce De León2017); Mexico (García-Vásquez et al. Reference García-Vásquez, Pinacho-Pinacho, Guzmán-Valdivieso, Calixto-Rojas and Rubio-Godoy2021), and Europe (e.g., Germany (Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970)), Croatia (Kiskaroly Reference Kiskaroly1977), Poland (Rokicka et al. Reference Rokicka, Lumme and Ziętara2007; Ziętara & Lumme Reference Ziętara and Lumme2004), and Serbia (Djikanovik et al. Reference Djikanovik, Paunovic, Nikolic, Simonovic and Cakic2012)). In England, the National Fisheries Services reported G. sprostonae as a cause of mass mortality of cultured carp, flagging it as an emerging pathogen and threat to fisheries (National Fisheries Services, www.gov.uk/environment-agency). Eight studies have provided morphometric data for G. sprostonae (Abdullah Reference Abdullah2013; Barzegar et al. Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018; Bykhovskaya-Pavlovskaya et al. Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964; Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Ling Reference Ling1962; Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970; Ogawa & Egusa Reference Ogawa and Egusa1978; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009).
The haptor of gyrodactylids comprizes four different sclerites; the hamulus, ventral bar, dorsal bar, and marginal hooks. The haptoral sclerites and the male copulatory organ (MCO), which lies below the pharynx, are used for Gyrodactylus species identification (Malmberg Reference Malmberg1970). There are seven sequences available for the internal transcribed spacer region of ribosomal DNA (ITS rDNA) of G. sprostonae. Of these sequences, only three (KP295469, AY278044, KT346368) span the entire ITS region of rDNA (Ziętara & Lumme Reference Ziętara and Lumme2004). Moreover, of all this data, only the sequence by Zietara & Lumme (Reference Ziętara and Lumme2004) relates to a peer-reviewed publication. The latter study supported the position of G. sprostonae in the subgenera Gyrodactylus (Limnonephrotus) Malmberg, 1964 using ribosomal DNA fragments. There is currently no record of G. sprostonae from Africa or the rest of the southern hemisphere. This study, therefore, aimed to incorporate traditional and modern techniques to study the gyrodactylids collected from L. aeneus in the Vaal River, South Africa, and additional morphometric and molecular data for the taxon.
Materials and methods
Sample collection
A total of 40 smallmouth yellowfish, L. aeneus (0.12–1.59 kg), were collected in March 2022 using gill nets at two sites (20 fish per site) along the Vaal River in Gauteng, South Africa in accordance with the conditions of permit CPE2 0118 and ethical clearance from the University of Johannesburg (03 May 2016). Site 1 was below the Vaal Dam (26°52′12.38″S; 28° 7′13.99″E), and Site 2 was below the Vaal River Barrage (26°44′6.26″S; 27°38′4.73″E) (Figure 1). The skin of fish was checked for monogenean parasites by scraping the skin with a glass microscope slide and examined for parasites using a Zeiss Stemi 350 stereomicroscope (Carl Zeiss, Germany). Thereafter, fish were euthanized by severing the spinal cord according to the South African National Standard: Care and Use of Animals for Scientific Purposes (2008). The gills and fins were dissected and examined for monogenean parasites using a Zeiss Stemi 350 stereomicroscope. Parasites were collected with a micropipette and either stored in 96% ethanol (Sigma-Aldrich, Germany) for scanning electron microscopy (SEM) and molecular analysis or mounted fresh with glycerine ammonium picrate (GAP) (Malmberg Reference Malmberg1957) to study with light microscopy (LM) as described below.

Figure 1. Collection sites along the Vaal River where Labeobarbus aeneus (Burchell, 1822) specimens were collected. A - African continent with South Africa shaded; B - map of South Africa indicating the area of collection sites; C - section of Vaal River system showing two collection sites, orange dot Site 1, purple dot Site 2.
Infection statistics
The prevalence and 95% confidence interval (CI) of collected specimens from respective sites were calculated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997) using Microsoft Excel (Microsoft Corporation, USA).
Light microscopy
Twenty whole worms were individually placed on microscope slides in a small drop of water, and a coverslip was placed over the specimen with pressure to expose the sclerotized structures. The corners of the coverslip were affixed onto the slide with nail varnish, then excess water was removed from the sides by capillary action using filter paper. A drop of GAP was placed on the edge of the coverslip and left to diffuse slowly into the specimen. Lastly, nail varnish was used to seal the sides of the coverslip. The preparation process was observed using a Zeiss Stemi 350 stereomicroscope (Carl Zeiss, Germany). A Zeiss Axioplan 2 imaging light microscope with Axiovision 4.7.2 software was used to study the specimens and obtain light micrographs of the different haptoral sclerites as well as the MCO. Point-to-point measurements of the sclerites were made following Shinn et al. (Reference Shinn, Hansen, Olstad, Bachmann and Bakke2004). Additionally, body length and width were measured. All measurements (mean ± standard deviation (minimum–maximum)) were compared to those of G. sprostonae presented by Abdullah (Reference Abdullah2013), Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018), Bykhovskaya-Pavlovskaya et al. (Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964), Daghigh Roohi et al. (Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019), Ling (Reference Ling1962), Mattheis & Glaser (Reference Mattheis, Glaser and Wundsch1970), Ogawa & Egusa (Reference Ogawa and Egusa1978), and Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). Line drawings of haptoral sclerites and the MCO were constructed using CorelDRAW (Taylor & Karney Reference Taylor and Karney1990) and compared to those presented in the aforementioned studies, all of which were redrawn for comparison.
Scanning electron microscopy
Thirteen whole worms (six from the first site and seven from the second site) stored in 96% ethanol were transferred to Tris-EDTA (TE) buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.96) overnight, then the buffer was changed three times over one-hour intervals. Thereafter, individual worms were placed on concavity slides with 0.5μl of digestion buffer from the E.Z.N.A.® Tissue DNA kit (Omega Bio-Tek Inc., Georgia, USA). The digestion protocol of Nation (Reference Nation1983) and Dos Santos & Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2015) was followed but modified as in Maduenyane et al. (Reference Maduenyane, Dos Santos and Avenant-Oldewage2022) to release and isolate haptoral sclerites. Following isolation, the sclerites were dried in a desiccator, and digested soft tissue was stored in a refrigerator for molecular analysis. Once dried, the sclerites were coated with gold using an Emscope SC500 sputter coater (Quorum Technologies, Lewes, UK) and micrographs taken at 6 kV acceleration voltage with a TESCAN Vega 3 LMH SEM (Brno, Czech Republic). Axiovision 4.7.2 software was used to generate point-to-point measurements of obtained micrographs for comparison with LM data. IBM SPSS version 28 was used for statistical analysis of haptoral sclerites and body measurements. Levene’s test and histograms confirmed that the data were not normally distributed; therefore, the Kruskal-Wallis non-parametric test was conducted to test for significant differences between specimens and also between LM and SEM data.
Molecular analysis
Genomic DNA of the 13 specimens for which the sclerites were isolated was extracted using the E.Z.N.A.® Tissue DNA kit. The ITS rDNA was amplified with primer set; ITS1A (5’-GTA ACA AGG TTT CCG TAG GTG-3’) (Matejusová et al. Reference Matejusová, Gelnar, McBeath, Collins and Cunningham2001) and ITS2R (5’-TCC TCC GCT TAG TGA TA-3’) (Cunningham Reference Cunningham1997). The following PCR conditions were used; 5 min @ 95°C, 35 cycles for 1 min @ 95°C, 1 min @ 55°C, 2 min @ 72°C then 5 min @ 72°C. Successful amplicons were verified using 1% agarose gel infused with SafeViewTM Classic (Applied Biological Materials Inc., Richmond, Canada) and a SmartDocTM 2.0 gel visualization and smartphone imaging system (Accuris instruments, Edison, NJ, USA). Standard BigDye chemistry was used to sequence the amplicons with an ABI 3137 Automated Sequencer (Applied Biosystems, Foster City, CA, USA). Obtained sequences were checked, aligned, assembled, and edited if needed with Geneious Prime version 2019.1.1 (http://www.genious.com), then compared to other Gyrodactylus species on GenBank. The Basic Local Alignment Search Tool (BLAST) (Altschul et al. Reference Altschul, Gish, Miller, Myers and Lipman1990) was used to select sequences of Gyrodactylus species that were closest to data generated in the current study (online supplementary Table S1). Sequences from GenBank that did not cover 80% of the alignment of the ITS rDNA were excluded. MEGA 7 (Tamura et al. Reference Tamura, Stecher, Peterson, Filipski and Kumar2013) was utilized to compute genetic distances based on both uncorrected p-distances and number of base pair (bp) differences. Maximum likelihood (ML) and Bayesian inference (BI) approaches were used to construct phylogenetic topologies. The model selection tool in MEGA 7 was used to select the General Time Reversible model with Gamma distribution (5 categories (+G, parameter = 0. 3807)). A total of 1000 bootstrap replicates were used to assess the robustness of this topology. BEAST v2.5.0 (Bouckaert et al. Reference Bouckaert, Heled, Kühnert, Vaughan, Wu, Xie, Suchard, Rambaut and Drummond2014) with 10 million Markov chain Monte Carlo (MCMC) generations was used for BI analysis. Sequences generated from the present study were submitted to GenBank.
Results
Gyrodactylus sprostonae Ling, 1962
Type host: Carassius auratus (Linnaeus, 1758) and Cyprinus carpio Linnaeus, 1758
Type locality: Liaohe River, China
New locality: Vaal River, South Africa (26°44′6.26″S; 27°38′4.73″E) and (26°52′12.38″S; 28° 7′13.99″E)
New host: Labeobarbus aeneus (Burchell, 1822)
Infection site: Gills
ITS rDNA reference sequences: Thirteen sequences submitted to GenBank (Accession numbers: OQ685901–13)
Infection statistics
Gyrodactylid parasites were not found on the skin and fins of fish but were present on the gills. Only one fish of 20 was infected with G. sprostonae from Site 1, and the prevalence was 5% (CI = –4.55–14.55%). From Site 2, nine hosts of 20 were infected; thus, the prevalence was 45% (CI = 23.2–66.8%). Aggregation was observed; most infected fish had only one gyrodactylid, while two fish (one from each site) had close to or more than 200 worms.
Morphometry (Figures 2–4)
All specimens with elongated bodies, total length 301 ± 80 (202–435) and width 66 ± 20 (40–114). Anterior bilobed and posterior comprised of haptor armed with pair of hamuli (Figures 2a & b) 49.4 ± 3.3 (41.3–54.3) long, root 18.6 ± 2.1 (15.1–21.6), and shaft 39.7 ± 2.4 (34.2–42.5) long. Hamuli connected by thin dorsal bar (Figure 2a) 17 ± 1.5 (14.7–20.8) long and 1.4 ± 0.3 (0.9–1.9) wide. Ventral bar (Figures 2d & e) 19.4 ± 2.2 (13.8–25.5) long and 19.6 ± 1.1 (18–22.3) wide, lies between hamuli, beneath dorsal bar (Figure 2a). Ventral bar comprised of thick, sclerotized horizontal bar, 19.6 ± 1.1 (18–22.3) wide, with sunken mid-point 3.5 ± 0.6 (2.8–5.1) long. Ventral bar with short, V-shaped, central process (Figure 2e) on dorsal side, and 14.6 ± 1.3 (10.4–16.2) long semi-sclerotized membrane. Haptor with 16 marginal hooks (Figure 2c), 24.9 ± 1.4 (21–26.7) long, with 19.7 ± 1.5 (16–22) long shaft, 5.2 ± 0.5 (3.7—6) long sickle, and 4.6 ± 0.4 (3.5–5.7) long sickle aperture. Marginal hook sickle has 3.5 ± 0.3 (2.9–4.2) proximal width and 3.2 ± 0.3 (2.6–3.9) distal width. Marginal hooks lack instep. Marginal hook sickle toe 1.6 ± 1.9 (1.1–1.9) long.

Figure 2. Scanning electron micrographs of isolated haptoral sclerites of Gyrodactylus sprostonae Ling, Reference Ling1962 from the current study. A - ventral side of hamuli and dorsal bar (20 μm); B - dorsal side of hamulus (10 μm); C - marginal hook (5 μm); D - dorsal side of ventral bar (10 μm); E - ventral side of ventral bar (5 μm).
hamuli (ha), dorsal bar (db), ventral bar membrane (vbm) v-shaped spike (vp).
The MCO (Figure 4) is bulbous, muscular, and armed with one large spine and six small spines. Four spines near the middle are smaller than the two lateral spines. There was no statistically significant difference between point-to-point haptoral measurements (Table 2) of specimens from the two study sites (Kruskal-Wallis p ˃ 0.05) based on LM observations. There was a statistically significant difference between the following LM and SEM point-to-point measurements: the hamulus total length (Kruskal-Wallis p = 0.006), point curve angle (Kruskal-Wallis p < 0.001), proximal shaft width (Kruskal-Wallis p < 0.001), inner aperture angle (Kruskal-Wallis p < 0.001), dorsal bar total length (Kruskal-Wallis p = 0.008), marginal hook sickle length (Kruskal-Wallis p = 0.009), sickle proximal width (Kruskal-Wallis p < 0.001), and toe length (Kruskal-Wallis p < 0.001). For the remaining 16 point-to-point measurements, there was no significant difference (Kruskal-Wallis p ˃ 0.05).
Table 2. Measurements (μm) of Gyrodactylus sprostonae Ling, Reference Ling1962 from the present study and all other available studies based on light and scanning electron microscopy.
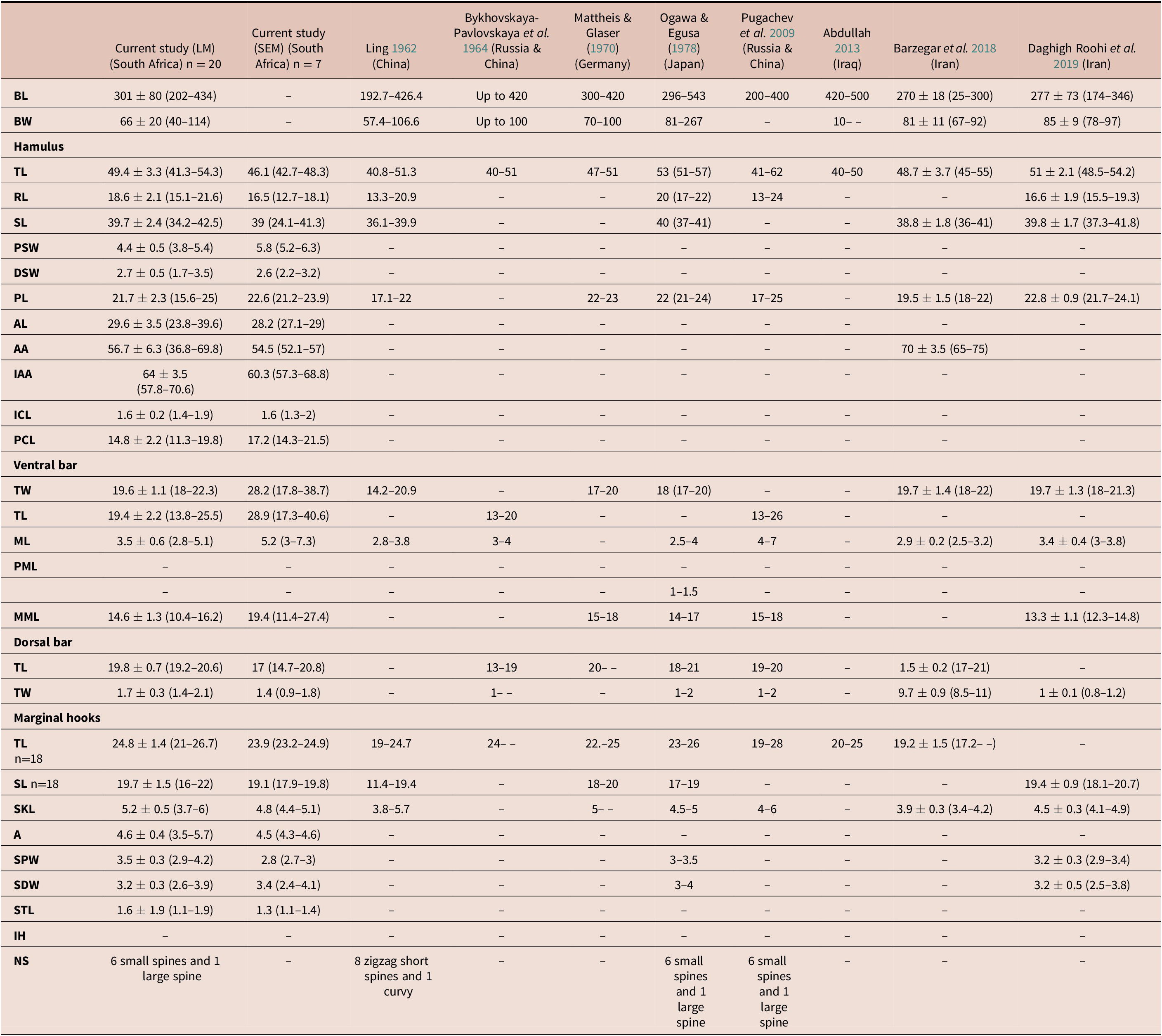
BL - Body length; BW - body width; TL - total length; RL - root length; SL - shaft length; PSW - proximal shaft width; DSW - distal shaft width; PL - point length; AL - aperture length; AA - aperture angle; IAA - inner aperture angle- ICL - inner curve length; PCA - point curve angle;; TW - total width; ML - median length; PML - process to mid-length; PML - process length; MML - Membrane length; SKL - sickle length; A - aperture; SPW - sickle proximal width; SDW - sickle distal width; STL - sickle toe length; IH - instep height; NS - number of spines
Key: (–) no measurement
(n) number of measured specimens
Remarks
Using the species identification key by Bykhovskaya-Pavlovskaya et al. (Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964) and Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009), the specimens from the current study represent G. sprostonae. Moreover, the morphology of the specimens from the present study was highly similar to the original description of G. sprostonae by Ling (Reference Ling1962). Morphology of the haptoral sclerites from the current study (Figure 3a) were similar to those of G. sprostonae from previous studies (Abdullah Reference Abdullah2013; Bykhovskaya-Pavlovskaya et al. Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964; Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Ling Reference Ling1962; Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970; Ogawa & Egusa Reference Ogawa and Egusa1978; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009) except for those by Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018). The ventral bar membrane was either absent or not detailed in most studies. The marginal hook sickle was most similar to the illustration of Bykhovskaya-Pavlovskaya et al. (Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964) (Figure 3l), Mattheis & Glaser (Reference Mattheis, Glaser and Wundsch1970) (Figure 3m), Ogawa & Egusa (Reference Ogawa and Egusa1978) (Figure 3n), Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009) (Figure 3o), and Daghigh Roohi et al. (Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019) (Figure 3r). Comparing sclerite and available MCO morphology of Gyrodactylus species reported from South African freshwater fishes to specimens from this study, there were no matches.

Figure 3. Illustrations of haptoral sclerites of Gyrodactylus sprostonae Ling, Reference Ling1962 from A, J - present study; B, K - Ling (Reference Ling1962); C, L - Bykhovskaya-Pavlovskaya et al. (Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964); D, M - Mattheis & Glaser (Reference Mattheis, Glaser and Wundsch1970); E, N - Ogawa & Egusa (Reference Ogawa and Egusa1978); F, O - Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009); G, P - Abdullah (Reference Abdullah2013); H, Q - Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018) and I, R - Daghigh Roohi et al. (Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019).
A-I - hamuli, dorsal and ventral bar (20 μm); J-R - marginal hook sickle (5 μm).

Figure 4. Illustrations of the male copulatory organ of Gyrodactylus sprostonae Ling, Reference Ling1962 (5 μm). A - MCO from the present study; compared to MCO from B - Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009); C - Ogawa & Egusa (Reference Ogawa and Egusa1978) and D - Ling (10962).
Genetic characterization
Identical sequences of ITS rDNA (1067–1163 bp) were obtained from all thirteen specimens (from both sites) in this study; thus only a single representative haplotype sequence was included for analyses. The alignment with selected Gyrodactylus data from GenBank was 1390 bp with 762 bp conserved, 624 bp variable, and 571 bp parsimony informative sites. The ITS rDNA phylogeny using both ML and BI showed that the sequences generated in the current study and sequences for G. sprostonae form a well-supported monophyletic group (Figure 5). A sister clade to the latter grouping comprized G. kobayashii and Gyrodactylus gurleyi Price, 1937. The intraspecific range for all included taxa for which more than one sequence is available was 0–0.07% (0–84 bp), while the interspecific range was 0–0.27% (6–327 bp) (excluding Gyrodactylus pomeraniae x lavareti hybrids) (online supplementary Table S2). Three sequences of G. sprostonae were included in the analysis and two sequences (KP295469 & AY278044) were identical to sequences generated in the present study (0%; 1–4 bp). The third sequence (KT346368) was 0.01% (7 bp) different from the data obtained here and other G. sprostonae data.

Figure 5. Combined maximum likelihood (ML) and Bayesian inference (BI) phylogenetic topology based on ITS rDNA analysis of Gyrodactylus sprostonae Ling, Reference Ling1962 and selected Gyrodactylus species based on BI. Statistical support for respective methods is indicated at branch nodes (BI/ML); nodes with less than 50% support are indicated with dashes. Data generated in the present study are in pink.
Discussion
This study presents the first record of G. sprostonae in the southern hemisphere and from a new, indigenous host, L. aeneus. The parasite was previously reported from indigenous and invasive hosts across the northern hemisphere (e.g., Abdullah Reference Abdullah2013; Barzega et al. Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018; Bykhovskaya-Pavlovskaya et al. Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964; Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Ling Reference Ling1962; Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970; Mhaisen & Abdul-Ameer Reference Mhaisen and Abdul-Ameer2013; Ogawa & Egusa Reference Ogawa and Egusa1978; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009), mostly from its type hosts, C. carpio and C. auratus (e.g., Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Ling, Reference Ling1962; Mendoza-Garfias et al. Reference Mendoza-Garfias, García-Prieto and Pérez-Ponce De León2017; Ogawa & Egusa Reference Ogawa and Egusa1978). Cyprinus carpio has been reported as a host of several invasive monogenean parasites in the Vaal River system such as G. kherulensis, Dactylogyrus extensus Mueller & Cleave, 1932 and Dactylogyrus minutus Kulweic, 1927 (Crafford et al. Reference Crafford, Luus-Powell and Avenant-Oldewage2014b). In a published conference abstract, Maseng et al. (Reference Maseng, Christison and Griffiths2009) reported two invasive Gyrodactylus species, G. kobayashii and G. kherulensis, from C. auratus and C. carpio respectively. However, the locality of the fish hosts was not mentioned in the abstract, but it is mentioned in an unpublished Master of Science study by Maseng (Reference Maseng2010). The latter stated that the fish were purchased from importers (imported from Asian and European countries), retailers and local breeders in Kuils River, South Africa. These records continue to remain uncertain as they have not been published in a peer-reviewed journal article. The presence of G. sprostonae on L. aeneus in the Vaal River system is suspected to be a recent introduction as it was not reported in previous monogenean surveys (Crafford et al. Reference Crafford, Luus-Powell and Avenant-Oldewage2012; Reference Crafford, Luus-Powell and Avenant-Oldewage2014a & Reference Crafford, Luus-Powell and Avenant-Oldewageb) conducted in this system. Furthermore, L. aeneus has been reported to host other invasive parasites, namely Schyzocotyle acheilognathi (Yamaguti, 1934) (Bertasso & Avenant-Oldewage Reference Bertasso and Avenant-Oldewage2005; Stadtlander et al. Reference Stadtlander, Weyl and Booth2011), Atractolytocestus huronesis Anthony, 1958 (Dos Santos & Avenant-Oldewage Reference Dos Santos and Avenant-Oldewage2022) and Argulus japonicus Thiele, 1900 (Kruger et al. Reference Kruger, Van As and Saayman1983; Tam & Avenant-Oldewage Reference Tam and Avenant-Oldewage2006; Van As & Basson Reference Van As and Basson1984;). The infection of L. aeneus with A. huronensis has also only recently been recorded, supporting Dos Santos & Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2022) who speculated that a recent introduction of carp to the Vaal River system may have taken place. It is likely that G. sprostonae infected L. aeneus through host-switching from non-native host species that are present in the Vaal River such as C. auratus, C. carpio and C. idella.
Comparing morphometry of G. sprostonae presented here to that from previous studies (Table 2), differences were observed. The body length and width of specimens from the present study and all previous studies overlapped, excluding Abdullah (Reference Abdullah2013). The total and shaft length of the hamuli correlated with those in other studies, while the root length overlapped with that of Ogawa & Egusa (Reference Ogawa and Egusa1978) but was longer than in all the other studies. Only Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018) measured the hamulus aperture angle, which was larger than in the current study. The hamulus point length was shorter in Ling (Reference Ling1962) and Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009) and overlapped with measurements presented by Mattheis & Glaser (Reference Mattheis, Glaser and Wundsch1970), Ogawa & Egusa (Reference Ogawa and Egusa1978), Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018), and Daghigh Roohi et al. (Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019). The hamulus proximal and distal shaft width, inner curve and aperture length, inner aperture angle, and point curve angle are presented for the first time for G. sprostonae in the present study. The total length of the ventral bar matched that of Ogawa & Egusa (Reference Ogawa and Egusa1978), Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018) and Daghigh Roohi et al. (Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019). The ventral bar of specimens in the present study corresponded with all previous studies but was wider than measurements in Ling (Reference Ling1962). Its median length was in range of other studies except that of Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). Only four previous studies measured the ventral bar membrane length (Egusa Reference Ogawa and Egusa1978; ; Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970; Ogawa & Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). The membrane length agreed with that by Ogawa & Egusa (Reference Ogawa and Egusa1978) and Daghigh Roohi et al. (Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019) but was shorter than those of Mattheis & Glaser (Reference Mattheis, Glaser and Wundsch1970) and Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). All specimens from the current and previous studies lack a ventral bar process, except for those studied by Ogawa & Egusa (Reference Ogawa and Egusa1978). The dorsal bar length corroborated measurements from previous studies, except for those of Bykhovskaya-Pavlovskaya et al. (Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964). The dorsal bar width overlapped with all previous studies, except for those studied by Barzegar et al. (Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018). Four previous studies presented measurements for the shaft of the marginal hook, which was shorter in Ling (Reference Ling1962) and overlapped with all previous studies. The aperture and sickle toe length of the marginal hooks are measured for the first time in this study.
Illustrations of the hamuli and dorsal bar were similar across all compared studies (Figure 3). However, there was variation in the morphology of the ventral bar. This sclerite was either excluded (Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019), not drawn in detail (Abdullah Reference Abdullah2013; Bykhovskaya-Pavlovskaya et al. Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964; Ling Reference Ling1962,), or shown to have a V-shaped (Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009) or U-shaped membrane (Barzegar et al. Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018; Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970; Ogawa & Egusa Reference Ogawa and Egusa1978). The ventral bar membrane from the present study is U-shaped with a narrow depression at the centre. This study and all previous studies, except for Ogawa & Egusa (Reference Ogawa and Egusa1978), agree that the horizontal rod of the ventral bar lack processes. The current study is the first to present a detailed illustration of the ventral bar, exposing a central V-shaped spike on the ventral side of this sclerite, following study with SEM. There was great variation in the comparison of the marginal hook sickle illustrations, as only five studies had a line drawing similar to that in the present study. This variation in illustrations is likely due to the sclerite morphology being altered by coverslip pressure or not flattened enough during LM preparation. It could also result from user error or incorrect identifications.
Using SEM for point-to-point haptoral sclerite measurements proved effective, as eight out of 24 measurements had statistically significant differences with LM measurements. This may be due to the technique allowing for the study of isolated sclerites, preventing alterations that could result from coverslip pressure in LM preparation as proposed by Mo & Appleby (Reference Mo and Appleby1990) and Shinn et al. (Reference Shinn, Gibson and Sommerville1993). This isolation of haptoral sclerites and examination using SEM has successfully been applied in several monogenean studies (e.g., Dos Santos et al. Reference Dos Santos, Dzika and Avenant-Oldewage2019a & Reference Dos Santos, Maina and Avenant-Oldewageb; Dos Santos & Avenant-Oldewage Reference Dos Santos and Avenant-Oldewage2015; Paladini et al. Reference Paladini, Huyse and Shinn2011; Shinn et al. Reference Shinn, Gibson and Sommerville1993; Tu et al. Reference Tu, Ling, Huang and Wang2015). It is likely that this technique is more accurate than LM, but it is not always comparable to previous studies that used only LM. As all previous studies used LM for morphological analysis of G. sprostonae (Abdullah Reference Abdullah2013; Barzega et al. Reference Barzegar, Ebrahimzadeh Mousavi, Rahmati-Holasoo, Taheri Mirghae and Bozorgnia2018; Bykhovskaya-Pavlovskaya et al. Reference Bykhovskaya-Pavlovskaya, Gusev, Dubinina, Izyumova, Smirnova, Sokolovskaya, Shtein, Shulman, Epshtein, Nagibina, Raikova and Strelkov1964; Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Ling Reference Ling1962; Mattheis & Glaser Reference Mattheis, Glaser and Wundsch1970; Ogawa & Egusa Reference Ogawa and Egusa1978; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009), the morphology of the ventral bar was either not detailed or excluded.
The elusive MCO was only presented in three studies (Ling Reference Ling1962; Ogawa & Egusa Reference Ogawa and Egusa1978; Pugachev et al. Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). The number of spines on the MCO corroborated the reports of Ogawa & Egusa (Reference Ogawa and Egusa1978) and Pugachev et al. (Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009), who counted six small and one large spine, but it is in contrast with the description by Ling (Reference Ling1962), who counted eight small, zigzagged spines and one large spine. It is suspected that the variation in the MCO may be due to the studied specimens being at different developmental stages, or that some of the soft tissue was misinterpreted for additional spines, as the number of small spines was consistent in all other studies. Even different preparation or microscopy techniques could produce different results. Several attempts to study the isolated MCO using SEM were unsuccessful here, as it could not be located after digestion.
Molecular characterization showed that specimens from the current study were identical or showed low intraspecific variability to available G. sprostonae ITS rDNA data, supporting the morphological analysis. The three G. sprostonae sequences that were compared to the haplotype from the current study are from different hosts and geographic regions in the northern hemisphere. Two of these sequences (KP295469 and AY278044) are identical to the haplotype from the Vaal River. They were collected from C. auratus in central China (unpublished study) and Poland (Ziętara & Lumme Reference Ziętara and Lumme2004), respectively. The third sequence (KT346368) was slightly different from the rest (0.01%; 7 bp), but the specimen for this sequence was collected from a different host, Hypophthalmichthys nobilis (Richardson, 1845), in western China (unpublished study). The genetic similarity between the sequences from C. auratus and those from the present study could support that G. sprostonae was co-introduced to the Vaal River alongside C. auratus. Phylogenetic topologies showed low support for the split node separating G. sprostonae sequences and the G. kobayashii and G. gurleyi cluster. These species parasitise similar hosts and have closely comparable morphological traits, thus supporting their phylogenetic proximity.
Labeobarbus aeneus is listed as a species of “least concern” by the International Union for Conservation of Nature (IUCN). It is endemic and widely distributed across the Orange-Vaal River system in South Africa but has been locally translocated through inter-basin water transfer schemes and for angling purposes (Skelton 2001). As a result, it is now present in the Limpopo and Olifants Rivers and several rivers of the Cape, such as Kei, Great Fish, Gourits, Sundays, and Kariega (Skelton 2001). Considering the loose host specificity of G. sprostonae, it is possible that this parasite may soon infect other indigenous hosts in the Orange-Vaal River system such as Labeobarbus kimberleyensis (Gilchrist & Thompson, 1913). The latter species is listed as near threatened under criteria B2b (ii.iii.v) by the IUCN. The prevalence of G. sprostonae, from the current and previous studies (Abdullah Reference Abdullah2013; Daghigh Roohi et al. Reference Daghigh Roohi, Dalimi Asl, Pourkazemi, Ghasemi and Shamsi2019; Ling Reference Ling1962), is mostly below 35%, with the exception of one instance reported by Ling (Reference Ling1962) where the prevalence was 54.4%. Irrespective of the latter, this parasite has been reported to cause mass mortality of cultured carp in well-managed fisheries (National Fisheries Services, www.gov.uk/environment-agency), and thus may pose a threat to indigenous hosts.
To conclude, this study not only presents additional taxonomic data, a new locality, and host switching to a new host for G. sprostonae, but also the first SEM of isolated sclerites of this parasite. Generated morphometric and molecular data contribute to the existing literature about this emerging invasive pathogen. Moreover, generated taxonomic information for this parasite may be utilized in further studies to track the source and spread of its invasive hosts in South African freshwater systems and globally.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0022149X23000202.
Acknowledgements
The authors thank the members of the Parasitology laboratory at the University of Johannesburg (UJ) for sampling assistance, Ms. Latiff for assistance with QGIS and SPSS, as well as the Spectrum Central Analytical Facility at UJ for the use of equipment and facilities. The authors would also like to thank the owners of the property below the Vaal Dam for access to the Vaal River and the use of their facilities.
Author contribution
Conceptualization: A.A.O, Q.M.D.S; Investigation: M.M:A.A.O., Q.M.D.S. Methodology: M.M, Q.M.D.S; Supervision: A.A.O, Q.M.D.S; Writing – review & editing: M.M, Q.M.D.S, A.A.O; Writing – original draft: M.M, Q.M.D.S, A.A.O; Formal analysis: M.M, Q.M.D.S, A.A.O.
Financial support
The National Research Foundation of South Africa provided a doctoral scholarship to M.M. The Oppenheimer Memorial Trust (2022-2023) granted post-doctoral fellowships to Q.MDS. The National Research Foundation and the University of Johannesburg (University Research Committee and Faculty Research Committee) provided funding for running the study and equipment expenses to A.A.O.
Competing interest
All authors declare that they have no conflict of interest.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.









