Introduction
The use of plant materials containing secondary metabolites (SMs) with in vivo anthelmintic (AH) activity has been proposed as an alternative method for the control of gastrointestinal nematodes (GINs) in veterinary medicine (Sandoval-Castro et al., Reference Sandoval-Castro, Torres-Acosta, Hoste, Salem and Chan-Pérez2012). The selection of plant materials with potential AH activity implies testing several plant extracts obtained through different extraction procedures. Likewise, the AH activity of those plant extracts should be assessed using validated tests (Hoste et al., Reference Hoste, Torres-Acosta, Sandoval-Castro, Mueller-Harvey, Sotiraki, Louvandini, Thamsborg and Terrill2015). In recent decades, the evaluation of the AH activity of plant extracts against GIN eggs has been based on an adaptation of the egg hatch test (EHT), which uses thiabendazole to kill eggs at the morula stage (Coles et al., Reference Coles, Bauer, Borgsteede, Klei, Taylor and Waller1992). The EHT is an easy to implement and economical tool that is used worldwide to screen the in vitro AH activity of plant materials (Jackson & Hoste, Reference Jackson, Hoste, Vercoe, Makkar and Schlink2010). In recent years, several studies showed that extracts of different Annona species have an ovicidal activity against Haemonchus contortus eggs. For instance, the methanol:water extract (70:30) of Annona squamosa seeds produced >80% egg-hatch inhibition (Souza et al., Reference Souza, Belvi, Morais, Costa, Silva and Braz-filho2008). Similarly, the methanol extract from leaves and bark also produced 100% egg-hatch inhibition (Kamaraj & Rahuman, Reference Kamaraj and Rahuman2011; Kamaraj et al., Reference Kamaraj, Rahuman, Elango, Bagavan and Zahir2011). More recently, an aqueous extract from A. muricata leaves was assessed showing 84% egg-hatch inhibition (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013). Those studies provided important information about the quantity of extract necessary to inhibit H. contortus egg hatching but failed to indicate the mechanism affecting egg hatching. Recent in vitro studies evaluating crude plant extracts from different plant species against H. contortus eggs revealed two processes explaining the activity related to different mechanisms: (a) death of eggs at the morula stage, also known as ovicidal activity; and (b) the inability of well-developed larvae to hatch from their egg shell, also known as larvae failing eclosion (LFE) (Vargas-Magaña et al., Reference Vargas-Magaña, Torres-Acosta, Aguilar-Caballero, Sandoval-Castro, Hoste and Chan-Pérez2014). Recent studies showed that tropical plant extracts inhibit egg hatching by affecting the ability of larvae to hatch from eggs (LFE activity), and very few plant materials show an ovicidal activity (Castañeda-Ramírez et al., Reference Castañeda-Ramírez, Torres-Acosta, Sandoval-Castro, González-Pech, Parra-Tabla and Mathieu2017, Reference Castañeda-Ramírez, Rodríguez-Labastida, Ortiz-Ocampo, González-Pech, Ventura-Cordero, Borges-Argáez, Torres-Acosta, Sandoval-Castro and Mathieu2018). However, those studies showed that methanolic extracts had higher ovicidal activity against H. contortus than the acetone-water extracts from the same plants. We hypothesize that methanolic extracts may allow to obtain more compounds associated with the ovicidal activity or may contain less compounds that antagonize with that activity. Such difference could be related to the polarity of extracts (Cortés-Morales et al., Reference Cortes-Morales, Olmedo-Juárez, Trejo-Tapia, González-Cortazar, Domínguez-Mendoza, Mendoza-de Gives and Zamilpa2019; García-Hernández et al., Reference García-Hernández, Rojo-Rubio and Olmedo-Juárez2019). Additionally, a recent study showed that the ovicidal activity was less evident for extracts with lower-condensed tannin content (Castañeda-Ramírez et al., Reference Castañeda-Ramírez, Torres-Acosta, Sandoval-Castro, González-Pech, Parra-Tabla and Mathieu2017). Thus, it is important to determine whether the use of different solvents in the extraction process (acetone:water vs. methanol) can influence the AH activity against nematode eggs. Such comparison can be used to investigate whether leaf extracts from different Annona species can affect H. contortus eggs at the morula stage. Furthermore, it is also important to identify the developing phase of eggs that is affected by each type of extract. The objective of this study was to assess and compare the AH activity of methanolic and acetone:water leaf extracts from different Annona species against H. contortus eggs.
Materials and methods
Location
This study was performed in the Faculty of Veterinary Medicine and Zoothecnics (FMVZ-UADY), Universidad Autónoma de Yucatán, Mérida, Mexico.
Biological material
Production of H. contortus eggs
The Paraiso H. contortus isolate was used for all the in vitro tests. This isolate was previously characterized as benzimidazole-resistant and as having low susceptibility to polyphenol-rich plant extracts (Chan-Pérez et al., Reference Chan-Pérez, Torres-Acosta, Sandoval-Castro, Hoste, Castañeda-Ramírez, Vilarem and Mathieu2016).
A four-month-old hair-sheep donor lamb (25 kg) raised free of GIN infections prior to the study was used to produce the H. contortus eggs. The donor lamb was fed a balanced diet based on grass hay, a commercial concentrate feed and water ad libitum. The donor was kept in an individual pen with raised slatted floors before and during the experiment and was inoculated with 4000 L3 of the isolate. The presence of eggs in the faeces was confirmed on day 28 post-infection. Faeces were collected three times a week directly from the rectum of the donor using plastic bags. Faeces were processed within 3 h of collection for the EHT. Egg recovery from faeces was carried out as follows: pellets were macerated in purified water, using 100 ml for every 10 g of faeces. The suspension was filtered using cheesecloth, and the filtered material was centrifuged (378 G for 5 min) in conical tubes (15 ml). Supernatant was discarded leaving sediment. A saturated sugar solution (1.28 density) was added and mixed with the sediment using a vortex to get a homogenous suspension. Suspension was centrifuged at 378 G for 5 min. A bacteriological loop was used to collect the supernatant of the suspension, where eggs were present. Eggs were placed in 15 ml tubes containing 10 ml of phosphate-buffered saline (PBS) pH 7.4. Egg concentration was determined, and suspension was diluted to 150 eggs/ml.
Production of methanolic and acetone:water extracts from leaves of Annona species
Fresh leaves from A. squamosa, A. muricata and A. reticulata (Annonaceae) were collected during the rainy season (October) in Yucatan, Mexico (20°56′N, 89°34′W). Specimens of each plant species were deposited in the FVMZ-UADY herbarium (voucher nos 14969, 14967 and 14968, respectively). The methanolic extracts were obtained from 500 g of fresh leaves from each plant species. Leaves were dried at 40°C for 72 h, until reaching a constant weight. Dried leaves were ground (1 mm particle size), weighed and placed in an individual container. Then, 30 ml of methanol was added for every 25 g of dried leaves. Samples remained under the organic solvent for 24 h, and this process was repeated once. The extract was recovered by filtration using filter paper (no. 50) and was concentrated under reduced pressure. Extracts were transferred to respective vials and placed in a laminar flow hood for 24 h to remove residual solvent. Finally, vials containing the respective extracts were closed and refrigerated at 4°C until further use in respective bioassays (Rosado-Aguilar et al., Reference Rosado-Aguilar, Aguilar-Caballero, Rodríguez-Vivas, Borges-Argaez, García-Vázquez and Méndez-González2010).
The acetone:water extracts were produced using 250 g of fresh leaves from each plant species. Fodder materials were crushed and placed in acetone:water (70:30) solution containing ascorbic acid (1 g/l) to avoid oxidation. The fodder materials were incubated for 24 h. Subsequently, the solution was recovered by filtration (filter paper no. 50). Acetone was removed under reduced pressure. The aqueous phase was rinsed twice with 500 ml methylene chloride to remove chlorophyll and lipids; thus, the remaining fraction was lyophilized and stored hermetically at 4°C until bioassays were conducted (Alonso-Díaz et al., Reference Alonso-Díaz, Torres-Acosta, Sandoval-Castro, Aguilar-Caballero and Hoste2008).
Assessment of the in vitro lethal effect of Annona spp. against H. contortus eggs
The EHT was used to evaluate the in vitro AH activity of the methanolic and acetone:water leaf extracts of the three Annona species against H. contortus eggs. The EHT was conducted following the procedure described by von Samson-Himmelstjerna et al. (Reference von Samson-Himmelstjerna, Coles and Jackson2009) and Jackson & Hoste (Reference Jackson, Hoste, Vercoe, Makkar and Schlink2010). Preparation of stock solutions (10 mg/ml) of extracts were made in PBS prepared with purified water plus 2% Tween-80 for methanolic extracts and only PBS for acetone:water extracts. A multi-well plate (24-wells) was used containing PBS and the respective volume of stock solution of extracts. The PBS + 2% Tween-80 and PBS were used as negative controls for the respective extracts. Subsequently, 1 ml of the H. contortus egg suspension (150 eggs/ml) was added to each well to obtain the final extract concentrations (150, 300, 600, 1200, 2400 and 3600 µg/ml). Six replicates were used for each extract concentration. The multi-well plates were placed in an incubator at 28°C. After 48 h of incubation, 100 µl of Lugol's solution was added to kill and dye eggs and larvae (L1). The number of eggs that failed to form larvae (morulated eggs (MEs)), the number of eggs that failed to complete their hatching (LFE) and the number of free larvae present in the sample were determined (Vargas-Magaña et al., Reference Vargas-Magaña, Torres-Acosta, Aguilar-Caballero, Sandoval-Castro, Hoste and Chan-Pérez2014).
To determine the role of condensed tannins and other polyphenols on the AH activity reported, solutions of different extracts were incubated with polyvinylpolypyrrolidone (PVPP; Fluka Analytical, Germany) (Makkar et al., Reference Makkar, Blümmel and Becker1995) (0.05 g of PVPP/ml of solution) for 3 h at 24°C. The PVPP was used as a polyphenol-blocking material. The PVPP is a polymer able to form a tannin capture net and it is commonly used to detect and quantify the total tannin content (Hernández-Bolio et al., Reference Hernández-Bolio, Kutzner, Eisenreich, Torres-Acosta and Peña-Rodríguez2018). After incubation, solutions were centrifuged at 1849 G for 5 min. The supernatant was used for testing at 3600 µg/ml, with and without PVPP, for the respective extracts in the same manner as described above. Six replicates were used for each extract concentration for the EHT.
Thin-layer chromatography (TLC)
The methanolic and acetone:water extracts from the Annona spp. were used to determine the metabolic profiles by TLC. Tests included extracts with and without PVPP incubation. The eluting system for TLC consisted of chloroform/MeOH/H2O + 50 µl of formic acid, using the following detection reagents: (a) phosphomolybdic acid for oxidizable compounds; (b) DPPH (1,1-diphenyl-2-picrilhidrazil) for phenolic compounds with antioxidant activity; (c) Kedde's reagent for acetogenins; and (d) Dragendorff's reagent for alkaloids. The ultraviolet visualization of TLC plates (254 and 365 nm) was also used to detect SM-containing chromophore groups (Jork, Reference Jork1990).
Data analysis
Means of MEs, eggs containing trapped larvae and hatched larvae were analysed through the Generalized Linear Model (GLM) analysis and compared with their respective controls. Post-hoc analysis was performed with Fisher's least significant difference (LSD) test, using Statgraphics Centurion XV software (Statpoint Technologies, 2005).
The extract activity on the assessed parameters – (a) MEs, (b) number of LFE and (c) the egg-hatching rate (EHR) – was determined using the following formulas (Chan-Pérez et al., Reference Chan-Pérez, Torres-Acosta, Sandoval-Castro, Hoste, Castañeda-Ramírez, Vilarem and Mathieu2016):
(1) The percentage of MEs was estimated as:
$${\rm \%\ ME} = \displaystyle{{{\rm Number}\;{\rm of}\;{\rm morulated}\;{\rm eggs}} \over {{\rm Number}\;{\rm of}\;{\rm morulated}\;{\rm eggs} + {\rm number}\;{\rm of}\;{\rm eggs}\;{\rm containing}\;{\rm a}\;{\rm larva} + {\rm number}\;{\rm of}\;{\rm larvae}}} \times 100$$
(2) The percentage of LFE was calculated as:
$${\rm \%\ LFE} = \displaystyle{{{\rm Number}\;{\rm of}\;{\rm eggs}\;{\rm containing}\;{\rm a}\;{\rm larvae}} \over {{\rm Number}\;{\rm of}\;{\rm morulated}\;{\rm eggs} + {\rm number}\;{\rm of}\;{\rm eggs}\;{\rm containing}\;{\rm a}\;{\rm larva} + {\rm number}\;{\rm of}\;{\rm larvae}}} \times 100$$
(3) The EHR expressed as percentage was calculated as:
$$\%\ {\rm EHR} = \displaystyle{{{\rm Number}\;{\rm of}\;{\rm larvae}} \over {{\rm Number}\;{\rm of}\;{\rm morulated}\;{\rm eggs} + {\rm number}\;{\rm of}\;{\rm eggs}\;{\rm containing}\;{\rm a}\;{\rm larva} + {\rm number}\;{\rm of}\;{\rm larvae}}} \times 100$$
Data for ME, LFE and EHR were compared with their respective control (PBS) and with the respective extract at 3600 µg/ml with or without PVPP. Data were analysed using a GLM procedure in each methanolic and acetone:water leaf extract from each Annona spp. For each parameter, a respective post-hoc analysis was performed with Fisher's LSD using Statgraphics Centurion XV software (Statpoint Technologies, 2005).
The effective concentration required to inhibit 50% of hatching (EC50) was estimated with data obtained from the EHR for each extract on the H. contortus eggs using the Polo-Plus 1.0 software (LeOra Software, 2004). The respective 95% confidence intervals (CIs) were also calculated. The EC50 values were considered significantly different when the 95% CIs did not overlap.
Results
Activity of Annona species leaf extracts against H. contortus eggs
Figure 1 shows the activity of methanolic and acetone:water leaf extracts of the three Annona species. The methanolic extracts of A. muricata (fig. 1a) and A. reticulata (fig. 1c) showed a significant reduction in egg hatching, starting from 300 µg/ml PBS, while the methanolic extract of A. squamosa only showed significant activity from 1200 µg/ml PBS (fig. 1e) (P < 0.05). The A. muricata and A. reticulata extracts showed activity against the morula stage of eggs from 600 µg/ml PBS. Meanwhile, the methanolic extract of A. squamosa showed activity against the morula stage from 1200 µg/ml PBS.

Fig. 1. Effect of different concentrations of methanol and acetone:water leaf extracts of Annona muricata, A. reticulata and A. squamosa on Haemonchus contortus egg-hatching inhibition. Graphs on the left correspond to methanolic extracts: (a) A. muricata; (c) A. reticulata; (e) A. squamosa. Graphs on the right correspond to acetone:water extracts: (b) A. muricata; (d) A. reticulata; (f) A. squamosa.
The acetone:water extracts of A. reticulata (fig. 1d) showed a significant reduction in egg hatching, starting at 600 µg/ml PBS, while the acetone:water extracts of A. muricata (fig. 1b) and A. squamosa (fig. 1f) only showed significant activities from 1200 µg/ml PBS (P < 0.05). The AH activity of acetone:water leaf extracts from Annona species was mainly related to block eclosion of larvae formed inside the egg (LFE activity), and a small proportion of eggs were killed at the morula stage.
Table 1 shows that the lowest EC50 values against the H. contortus eggs were found with the methanolic extracts of A. muricata and A. reticulata. The acetone:water extract of A. reticulata was the least active against H. contortus eggs.
Table 1. Effective concentration 50% (EC50) and respective 95% CIs of methanolic and acetone:water leaf extracts of Annona species against eggs of Haemonchus contortus.

a−eDifferent letters in the same column mean significant difference (P < 0.05).
The effect of PVPP on the assessed parameters (proportion of ME, LFE and hatched eggs) of H. contortus after exposure to methanolic and acetone:water leaf extracts is shown in table 2. Methanolic extracts of A. squamosa, A. muricata and A. reticulata showed high ovicidal activity (92.8–98.9% ME). Pre-incubation of the A. reticulata methanolic extracts with PVPP increased the proportion of ME to 99.0% (P < 0.05).
Table 2. Effect of the addition of PVPP on the proportion (%) of MEs, LFE and L1 of Haemonchus contortus resulting from incubations with methanolic and acetone:water leaf extracts from three Annona species at a concentration of 3600 µg/ml PBS.
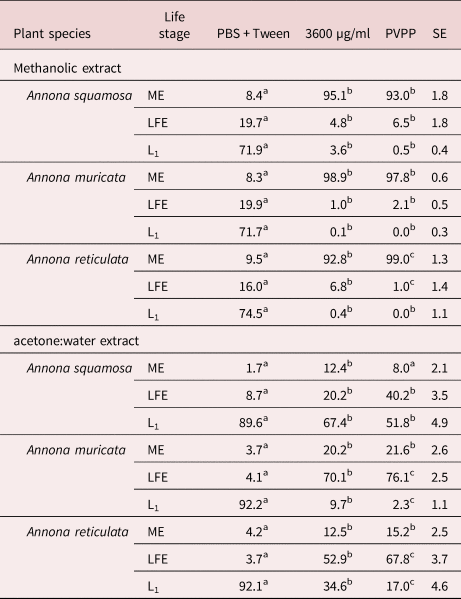
a–cDifferent letters in the same row mean significant difference (P < 0.05).
SE, standard error.
On the other hand, the acetone:water extracts showed low ovicidal activity(12.4–20.7% ME). The acetone:water extracts showed a high LFE activity against H. contortus eggs.
Lesions on H. contortus eggs and L1
The normal morula stage of eggs incubated in PBS is shown in fig. 2a, and the ovicidal activity of extracts is shown in fig. 2b. In the latter, it is evident that the egg contains more liquid in comparison to the egg exposed to PBS. Furthermore, the morula cells appeared shrunk and rough in the egg exposed to extracts, in contrast to the turgid morula cells of the egg in PBS.
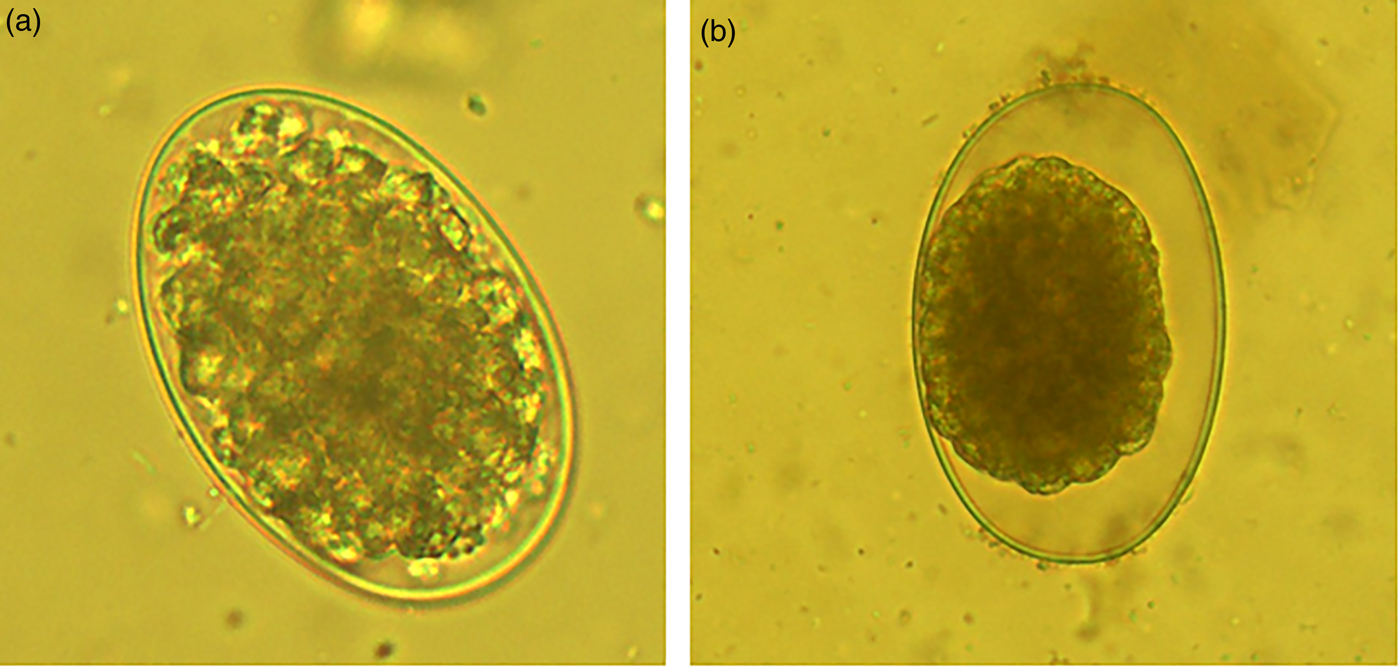
Fig. 2. Normal morulated Haemonchus contortus egg (a) exposed to PBS and (b) with a death morula resulting from the incubation with extracts of different Annona spp.
On the other hand, the acetone:water extracts damaged the larvae formed inside the egg. The larvae trapped inside eggs had a compressed aspect and wrinkled architecture (fig. 3a). Likewise, the few L1 that were able to hatch from eggs once exposed to acetone:water extracts were dead, and presented a generalized body swelling characterized by a separation of the shrivelled digestive tract, which seemed separated from the external cuticle compared to larvae exposed to PBS (see fig. 3b, c).
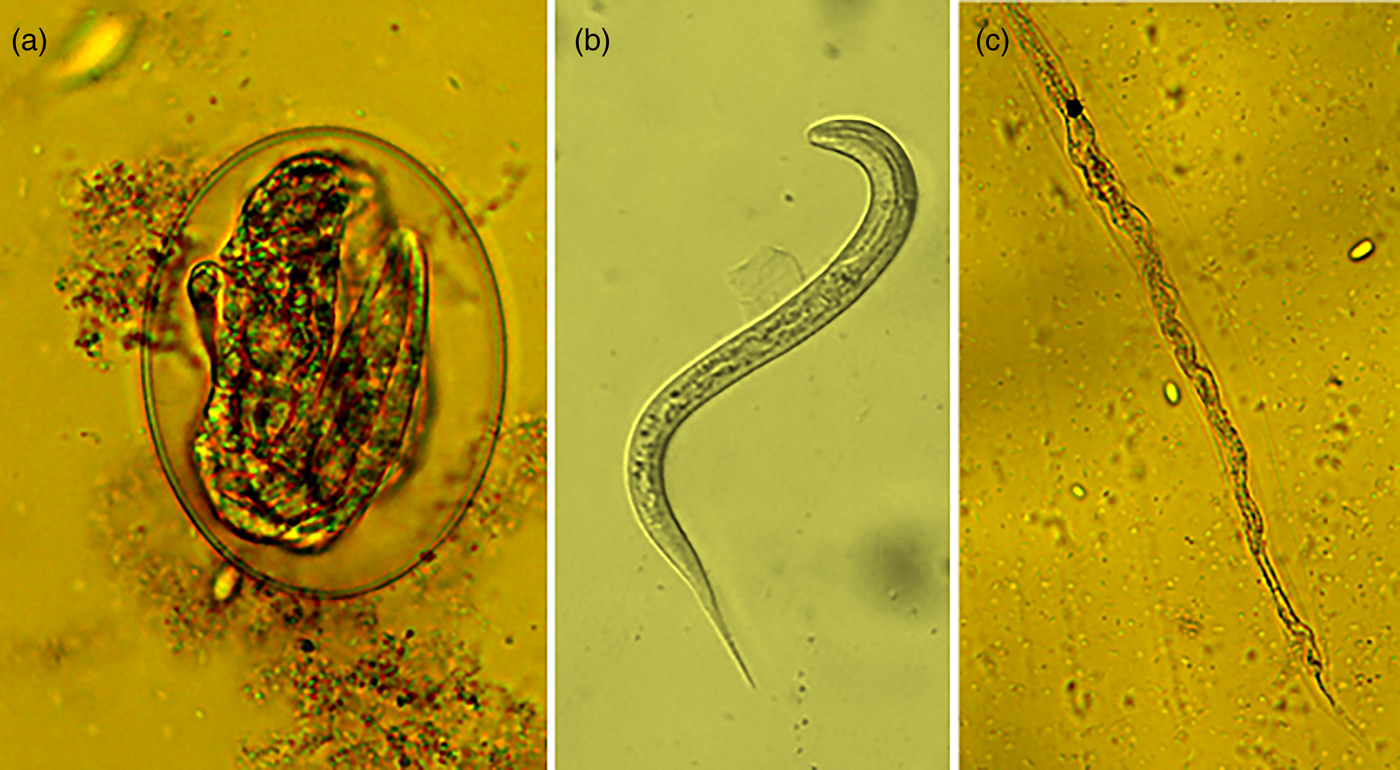
Fig. 3. (a) Haemonchus contortus egg incubated for 48 h in acetone:water extract of Annona spp. leaves showing a larva inside the egg; (b) image of a normal larva obtained from a control PBS incubation; (c) image of a deformed death larva that emerged from an egg exposed to acetone:water extracts.
Groups of compounds identified by the Thin Layer Chromatography
The methanolic extracts showed the presence of compounds with a wide polarity range (from non-polar to polar), while the acetone:water extracts revealed the presence of compounds with medium to high polarity. In spite of these findings, both extraction systems showed a similar profile of polar metabolites. Phosphomolybdic acid identified the presence of flavonoids in those leaf extracts not incubated with PVPP, while DPPH revealed the presence of phenolic compounds with antioxidant activity in the acetone:water extracts that was less evident in methanolic extracts. Non-phenolic compounds with antioxidant activity were detected in the PVPP-incubated extracts. The use of Kedde's reagent did not show evidence of acetogenins in the tested extracts, with or without PVPP incubation. Finally, Dragendorff's reagent suggested the presence of alkaloids, and that was only evident for the methanolic extracts, irrespective of the PVPP incubation.
Discussion
This study showed that the extracts obtained from leaves of the tested Annona species possess strong in vitro activity against H. contortus eggs. The latter confirm previous in vitro AH activity against H. contortus egg hatching obtained with extracts from different parts of plants of the Annonaceae family, as described below. In the present study, low concentrations of the methanolic plant extracts (2.4 mg/ml) were sufficient to achieve almost 100% egg-hatching inhibition (fig. 1). The activity of A. muricata and A. squamosa acetone:water extracts also reduced egg hatching >90% from a concentration of 3.6 mg/ml (fig. 1). In comparison, the lowest extract concentrations previously tested were reported for the methanol:water extract of A. squamosa seeds, where 5 and 2.5 mg/ml reduced egg hatching by 81.9% and 81.5%, respectively (Souza et al., Reference Souza, Belvi, Morais, Costa, Silva and Braz-filho2008). Further studies using acetonic, methanolic and ethyl acetate extracts of A. squamosa bark required 25 mg/ml to reduce egg hatching >90% (Kamaraj et al., Reference Kamaraj, Rahuman, Elango, Bagavan and Zahir2011). When using the same organic solvents for the leaves of A. squamosa, the egg-hatch inhibition was <88.6% at 25 mg/ml (Kamaraj & Rahuman, Reference Kamaraj and Rahuman2011). The last study reported an 84.9% egg-hatch inhibition for the A. muricata organic extracts, but the concentrations tested were not defined (Ferreira et al., Reference Ferreira, Castro, Chagas, França and Beleboni2013). The stronger AH activity of the Annona species tested in the present study could be attributed to different factors, such as the influence of environmental conditions in Yucatan, with a warm and sub-humid tropical climate, the plants' surrounding soil microbiota, the age of plants at collection time, the physiological stage of plants, micro-environmental conditions, sunlight exposure, soil water, fertility and salinity, damage caused by herbivores, among others factors, as all of those factors can influence the SM profile of plants (Badri et al., Reference Badri, Zolla, Bakker, Manter and Vivanco2013; Yang et al., Reference Yang, Wen, Ruan, Zhao, Wei and Wang2018). The latter could also affect their expected anti-parasitic activity (Arceo-Medina et al., Reference Arceo-Medina, Rosado-Aguilar, Rodríguez-Vivas and Borges-Argaez2016; Hoste et al., Reference Hoste, Torres-Acosta, Quijada, Chan-Pérez, Dakheel, Kommuru, Mueller-Harvey, Terrill, Gasser and von Samson-Himmelstjerna2016). The plant extraction procedure, including the drying of plant materials as well as the volume and type of solvents used, could also influence the concentration of bioactive metabolites causing the AH activity (Hoste et al., Reference Hoste, Torres-Acosta, Quijada, Chan-Pérez, Dakheel, Kommuru, Mueller-Harvey, Terrill, Gasser and von Samson-Himmelstjerna2016; Hernández-Bolio et al., Reference Hernández-Bolio, Kutzner, Eisenreich, Torres-Acosta and Peña-Rodríguez2018).
Extracts evaluated in the present study also showed stronger AH activity against H. contortus eggs compared to previous results with other plants species. For example, a methanolic extract obtained from Tagetes filifolia required 10 mg/ml to achieve 100% egg-hatching inhibition (Jasso-Díaz et al., Reference Jasso-Díaz, Hernández, Zamilpa, Becerril-Pérez, Ramírez-Bribiesca, Mernández-Mendo, Sánchez-Arrollo, González-Cortazar and Mendoza-de Gives2017). Likewise, 100 mg/ml of a methanol:water extract from Acacia cochliacantha leaves was required to achieve 100% egg-hatching inhibition (Castillo-Mitre et al., Reference Castillo-Mitre, Olmedo-Juárez and Rojo-Rubio2017). On the other hand, the tested Annona extracts showed similar AH activity to that of methanol:water and acetone:water extracts from several plant species of the tropical deciduous forest. For instance, methanol:water extracts obtained from Gymnopodium floribundum, Havardia albicans, Leucaena leucocephala, Mimosa bahamensis, Piscidia piscipula and Senegalia gaumeri leaves caused >98% egg-hatching inhibition at 3.6 mg/ml (Castañeda-Ramírez et al., Reference Castañeda-Ramírez, Torres-Acosta, Sandoval-Castro, González-Pech, Parra-Tabla and Mathieu2017). Also, the acetone:water extracts from the leaves of those plants and from Acacia collinsi, A. penatula and Bunchosia swartziana provoked 98% egg-hatching inhibition at 3.6 mg/ml (Castañeda-Ramírez et al., Reference Castañeda-Ramírez, Rodríguez-Labastida, Ortiz-Ocampo, González-Pech, Ventura-Cordero, Borges-Argáez, Torres-Acosta, Sandoval-Castro and Mathieu2018).
Most extracts produced from plants of the tropical deciduous forest inhibited egg hatching by blocking the larvae eclosion (LFE activity); however, the Annona leaf extracts showed strong ovicidal activity (methanolic extracts; fig. 2a, b), as well as LFE activity (acetone:water extracts; fig. 3a–c). Previous studies evaluating the AH activity of Annona extracts only reported an egg-hatching inhibition but failed to clarify whether that activity was directed against the morula stage, or was associated with an LFE activity. The strong ovicidal activity reported in the present study for the methanolic extracts of Annona leaves is an interesting target of AH activity.
The physiological mechanism causing the ovicidal activity with these plants extracts is currently unknown. The inhibition of embryonation of freshly collected nematode eggs (ovicidal activity) was observed for the commercial drug thiabendazole (Coles et al., Reference Coles, Bauer, Borgsteede, Klei, Taylor and Waller1992). Thiabendazole causes the inhibition of the fumarate reductase enzyme, which is specific to helminths (Prichard, Reference Prichard1973; Lacey, Reference Lacey1988).
The difference in AH activity between the methanol and the acetone:water extracts could be due to differences in the SM obtained with the two solvents. It is possible that the methanolic extracts have smaller SM, that could penetrate the eggshell, causing a higher lethal effect compared to SM present in acetone:water extracts. Additionally, the Dragendorff's reagent showed that the methanolic extracts contained alkaloids, which were not present in the acetone:water extracts. Those compounds could have an implication in the AH activity against the morula inside the egg.
Alkaloids present in A. muricata have cytotoxic and neurotoxic effects (Coria-Téllez et al., Reference Coria-Téllez, Montalvo-Gónzalez, Yahia and Obledo-Vázquez2018), and perhaps those compounds could cause death of eggs at the morula stage.
Meanwhile, the LFE activity could involve the presence of SM that are present in many plant species (Vargas-Magaña et al., Reference Vargas-Magaña, Torres-Acosta, Aguilar-Caballero, Sandoval-Castro, Hoste and Chan-Pérez2014). Different compounds affecting H. contortus egg hatching have been studied, including P-coumaric acid (Castillo-Mitre et al., Reference Castillo-Mitre, Olmedo-Juárez and Rojo-Rubio2017; Castañeda-Ramírez et al., Reference Castañeda-Ramírez, Rodríguez-Labastida, Ortiz-Ocampo, González-Pech, Ventura-Cordero, Borges-Argáez, Torres-Acosta, Sandoval-Castro and Mathieu2018). Such compounds obtained from polyphenol-rich plant extracts allowed L1 development inside the egg, but the eggshell could not be broken, similar to the egg shown in fig. 3a.
Blocking polyphenols with PVPP did not affect the AH activity of A. squamosa and A. muricata methanolic extracts (table 2), while the incubation with PVPP improved the AH activity of A. reticulata methanolic extract. The latter suggests that polyphenols were not involved in the AH activity of A. squamosa or A. muricate, but could limit the ovicidal activity of A. reticulata. The antagonism between the SM causing ovicidal activity and polyphenols contained in leaf extracts was already reported in previous studies (Vargas-Magaña et al., Reference Vargas-Magaña, Torres-Acosta, Aguilar-Caballero, Sandoval-Castro, Hoste and Chan-Pérez2014; Castañeda-Ramírez et al., Reference Castañeda-Ramírez, Torres-Acosta, Sandoval-Castro, González-Pech, Parra-Tabla and Mathieu2017).
The Annona leaf extracts evaluated in the present study warrant further investigation aiming to identify the active SMs causing the ovicidal activity. Meanwhile, these extracts can also help to identify compounds causing the LFE activity. Once these compounds are identified, commercial standards can be used to confirm those findings.
Conclusion
Methanol extracts obtained from leaves of A. muricata, A. reticulata and A. squamosa showed ovicidal activity affecting the morula stage of H. contortus eggs, with minor LFE activity. Meanwhile, the acetone:water extracts from the leaves of those same plants showed mostly LFE activity, with a smaller proportion of ovicidal activity.
Acknowledgements
The authors wish to express their gratitude to the following people: Guadalupe Ortiz, Rodrigo, Iris Trinidad, Pedro Gonzalez, Concepcion Capetillo, Dr. Luis Manuel Peña-Rodriguez and all of the team at the Small Ruminant Research Department, UADY.
Financial support
We acknowledge the financial support of Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), Mexico, (Proyecto Fiscal 2012 ‘Evaluación de cuatro extractos de plantas como fitoterapéuticos contra nematodos parásitos de ovinos’). G.S. Castañeda-Ramírez acknowledges the Master in Science scholarship obtained from Consejo Nacional de Ciencia y Tecnologia (CONACYT), México (number 336930).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. All procedures performed on donor animals complied with the Ethical Standards of the Bioethics Committee of the Faculty of Veterinary Medicine, UADY, Mexico.







