Introduction
Two large islands, Sakhalin and Hokkaido, are washed by the waters of the Sea of Okhotsk and the Sea of Japan; Kunashir Island of the Kuril Chain are in the waters of the Sea of Okhotsk and the Pacific Ocean. Moneron Island is located in the Sea of Japan (Figure 1). The uniqueness of these islands is due to their extreme longitudinal geographical position, the diverse topography, differences in climatic conditions, the presence of warm and cold sea currents, and geological history. The islands’ repeated connections to and separations from the mainland have been documented (Velizhanin, Reference Velizhanin1976; Millien-Parra & Jaeger, Reference Millien-Parra and Jaeger1999; Pietsch et al., Reference Pietsch, Bogatov, Amaoka, Zhuravlev, Barkalov, Gage, Takahashi, Lele, Storozhenko and Minakawa2003; Bogatov et al., Reference Bogatov, Pietsch, Storozhenko, Barkalov, Lelej, Kholin, Krestov, Kostenko, Makarchenko, Prozorova and Shedko2006). These factors determine the species diversity and geographic distribution of helminths of small mammals in this region.

Figure 1. Sampling sites of shrew cestodes on islands Sakhalin, Hokkaido, Kunashir, and Moneron: 1: Cape Lebedinyi, 2: Poronaysky Nature Reserve, 3: Shakhtersk, 4: Sokol Biological Station, 5: Korsakov, 6: Moneron Island, 7: Kunashir Island, 8: Meto, 9: Tomakomai.
Nine shrew species of the genus Sorex have been recorded in the south of the Russian Far East: Sorex caecutiens Laxmann, 1788, S. daphaenodon Thomas, 1907, S. gracillimus Thomas, 1907, S. isodon Turov, 1924, S. minutissimus Zimmermann, 1780, S. mirabilis Ognev, 1937, S. roboratus Hollister, 1913, S. tundrensis Merriam, 1900 and S. unguiculatus Dobson, 1890. Six of them inhabit Sakhalin Island: S. caecutiens, S. daphaenodon, S. gracillimus, S. isodon, S. minutissimus and S. unguiculatus) (Ochotina, Reference Ochotina1977). On Kunashir and on the Japanese island Hokkaido, there are four species of shrews (S. caecutiens, S. gracillimus, S. minutissimus, and S. unguiculatus) (Grigoryev & Bashilov, Reference Grigoryev and Bashilov1988; Kostenko et al., Reference Kostenko, Nesterenko and Trukhin2004; Ohdachi et al., Reference Ohdachi, Ishibashi, Iwasa, Fukui and Saitoh2015). On the small Moneron Island, only S. tundrensis is found (Ochotina, Reference Ochotina1976; Kostenko & Nesterenko, Reference Kostenko, Nesterenko and Storozhenko2006).
Parasitologically, shrews of Northeast Asia, in particular Eastern Siberia and the Far East, have been studied quite well (Odnokurtsev & Karpenko, Reference Odnokurtsev and Karpenko1993; Melnikova et al., Reference Melnikova, Gulyaev, Dokuchaev and Alekseev2003, Reference Melnikova, Gulyaev and Dokuchaev2005; Karpenko, Reference Karpenko2004; Kornienko et al., Reference Kornienko, Gulyaev, Melnikova and Georgiev2008a, Reference Kornienko, Zubova, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskijb, Reference Kornienko, Makarikov and Dokuchaev2014, Reference Kornienko, Dokuchaev and Odnokurtsev2018; Kornienko & Dokuchaev, Reference Kornienko and Dokuchaev2023); the same is not true about the shrews of the islands of the Sea of Okhotsk and the Sea of Japan. Data about shrew cestodes from Sakhalin, Kunashir and Hokkaido were sampled at the end of the last century and at the beginning of the current century. These were either faunal summaries of cestodes (Sato et al., Reference Sato, Kamiya and Ohbayashi1988; Sawada & Koyasu, Reference Sawada and Koyasu1991, Reference Sawada and Koyasu1995; Sawada & Asakawa, Reference Sawada and Asakawa1992; Sawada & Kaneno, Reference Sawada and Kaneno1992; Sawada & Kobayashi, Reference Sawada and Kobayashi1993; Karpenko, Reference Karpenko and Fedorov1997; Sawada, Reference Sawada1999) or descriptions of new species (Sawada & Harada, Reference Sawada and Harada1990; Sawada & Koyasu, Reference Sawada and Koyasu1991; Karpenko, Reference Karpenko1999; Lykova et al., Reference Lykova, Melnikova and Karpenko2005, Reference Lykova, Gulyaev, Melnikova and Karpenko2006; Zubova et al., Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008a, Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Movsessian, Be′er, Zinovieva, Pelgunov and Spiridonovb; Kornienko et al., Reference Kornienko, Gulyaev, Melnikova and Georgiev2008a, Reference Kornienko, Makarikova and Dokuchaev2023). This resulted in the assumption of high species diversity of shrew cestodes on these islands. The main objective of those studies was to inventory the available collections from the previously mentioned islands and to update the information in view of the modern concepts in taxonomy of the cestode families Hymenolepididae and Dilepididae (Gulyaev et al., Reference Gulyaev, Kornienko and Dokuchaev2007; Kornienko et al., Reference Kornienko, Gulyaev and Melnikova2006, Reference Kornienko, Gulyaev and Melnikova2007, Reference Kornienko, Gulyaev, Melnikova and Georgiev2008a, Reference Kornienko, Binkienė and Tkach2016, Reference Kornienko, Binkienė, Dokuchaev and Tkach2019, Reference Kornienko and Dokuchaev2023; Binkienė et al., Reference Binkienė, Kornienko and Tkach2015; Neov et al., Reference Neov, Vasileva, Radoslavov, Hristov, Littlewood and Georgiev2019, Reference Neov, Vasileva, Radoslavov, Hristov, Littlewood and Georgiev2021). Other interesting questions are associated with the comparison of the cestode fauna of mainland and islands, the dependence of the cestode fauna on the host-species diversity and abundance, as well as the role of the size of the islands and their remoteness from the continent for the parasite diversity.
The purpose of the present study is to analyze the data about the species diversity of cestodes parasitizing shrews on the islands in the Sea of Okhotsk and the Sea of Japan (Sakhalin, Kunashir, Hokkaido and Moneron). We present a comparative analysis of the taxonomic composition of shrew cestodes across the islands and the mainland. This allows us to identify cestode species shared among islands (or mainland) or endemic to certain islands as well as to study the effects of the size and remoteness of the islands and the diversity of hosts on the cestode diversity.
Materials and Methods
We carried out a helminthological study on shrews in the summer of 2005–2007 on the islands Sakhalin, Kunashir, Moneron, and Hokkaido (Figure 1).
On Sakhalin, shrews were analyzed in a northern part of the island (Cape Lebediniy, N 53°09′, E 143°13′), a central part of the island (Poronaysky Nature Reserve, N 49°13′ E 143°06′, and Shakhtersk town N 49°09′, E 142°06′) as well as in the southern part of the island (Sokol Biological Station [SBS], N 47°14′, E 142°46′, and environs of the towns Korsakov N 46°46’, E 143°00’ and Yuzhno-Sakhalinsk, N 47°01′, E 142°43′). We examined for cestodes 316 individuals of five species: S. unguiculatus, S. gracillimus, S. caecutiens, S. minutissimus, and S. daphaenodon.
On Kunashir Island, the materials were collected at cordons of the Kuril Nature Reserve (Rudniy N 44°22′, E 146°00′, Andreyevskiy N 43°53′, E 145°37′, Filatovskiy, N 44°11′, E 146°01′, and Ozerniy, N 43°52′, E 145°28′) in June–July 2006. We examined 119 individuals of three species for cestodes: S. unguiculatus, S. gracillimus, and S. caecutiens.
On Moneron Island (N 46°16′, E 141°14′) in 2005, only two individuals of S. tundrensis were captured and examined.
On Hokkaido Island, the specimens were collected in August 2005 in a central part (Meto: N 43°23′, E 143°20′) and a southwestern part (Tomakomai: N 42°37′, E 141°45′). We examined for cestodes 69 shrews: S. unguiculatus, S. caecutiens, and S. gracillimus.
Data on the number of shrew individuals studied are given in Table 1.
Table 1. Numbers of studied shrews (Sorex) from islands Sakhalin, Kunashir, Hokkaido, and Moneron

In addition, we reexamined the collection of cestodes from shrews sampled in 1989 by S.V. Karpenko in the Kuril Nature Reserve (Kunashir Island) in the vicinity of Golovnino Village (N 43°44′, E 145°31′) (Karpenko, Reference Karpenko and Fedorov1997).
To compare species composition of cestodes among shrew species, we used the Jaccard Similarity Coefficient (Cj) (Magurran, Reference Magurran1988): Cj = j/(a + b – j), where a is the number of species in the first set being compared, b is the number of species in the second compared set, and j is the number of species common between the two sets.
Results
In the shrews inhabiting islands Sakhalin, Kunashir, Hokkaido and Moneron, 36 species of cestodes were recorded. They belonged to three families Hymenolepididae Perrier, 1897, Dilepididae Fuhrmann, 1907, and Mesocestoididae Perrier, 1897. Among the studied islands, differences were found in both species diversity and number of taxa. Shrews from Sakhalin were parasitized by 33 cestode species, whereas those from Kunashir had 22, and those from Hokkaido were hosts to 23 species (Table 2). In two S. tundrensis individuals caught on Moneron Island, only three species of cestodes were detected (M. baicalensis, N. nadtochijae, and S. furcatа). The species Dilepis undula and Mesocestoides sp. were registered as being in larval stages only. D. undula larvae were found in the intestine. D. undula is a parasite of birds, and shrews are considered as its abortive host. Larval forms of Mesocestoides sp. (parasitic as adults in intestines of carnivore mammals and birds of prey) occurred in the body cavity of shrews.
Table 2. The occurrence of shrew cestodes in the south of the Far East of Russia and on islands Sakhalin, Kunashir, and Hokkaido
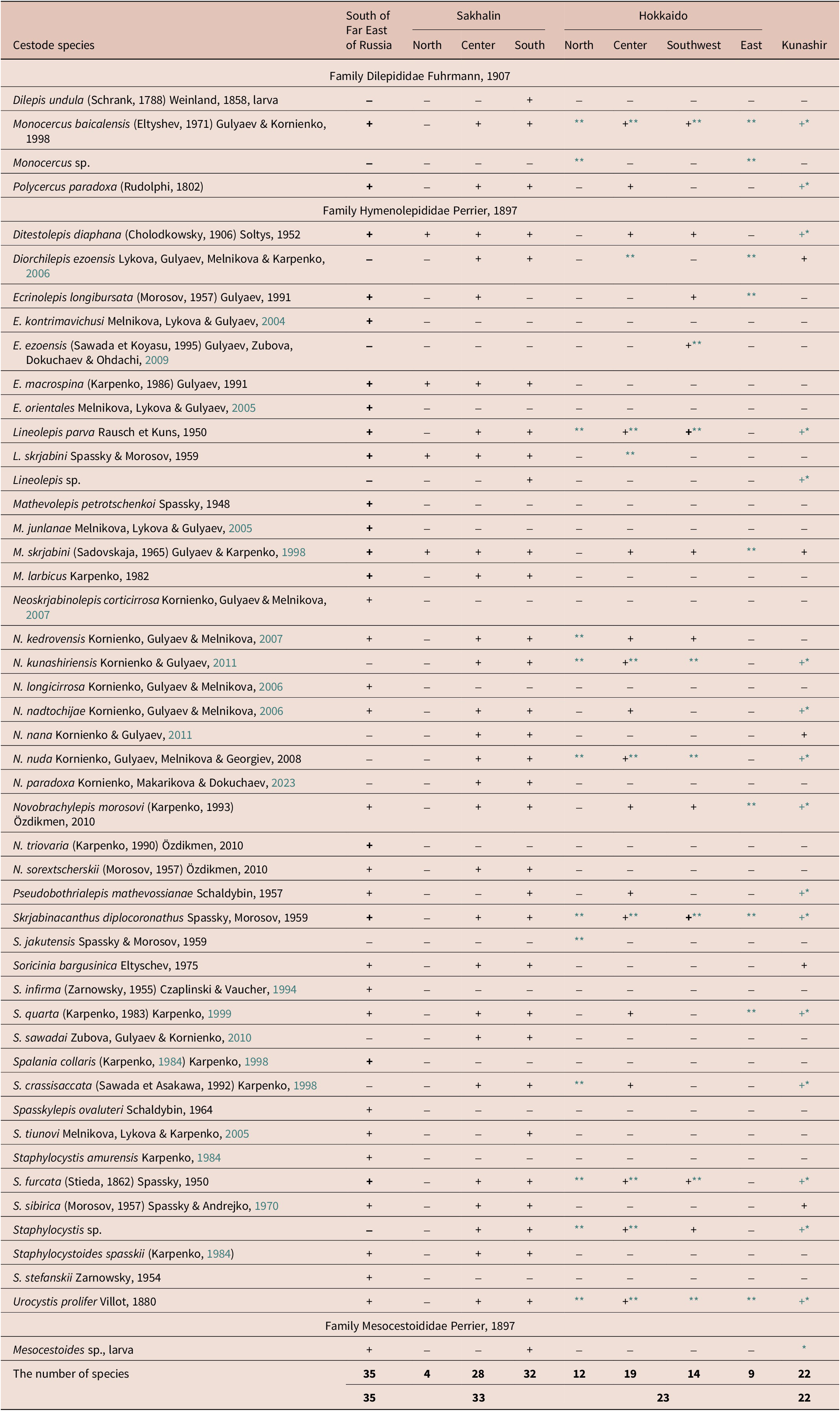
* Shrew cestodes of Kunashir Island from Karpenko’s collection (1997).
** Species of shrew cestodes from Hokkaido Island that have been found by Japanese authors (Sato et al., Reference Sato, Kamiya and Ohbayashi1988; Sawada & Koyasu, Reference Sawada and Koyasu1991, Reference Sawada and Koyasu1995; Sawada & Asakawa, Reference Sawada and Asakawa1992; Sawada & Kaneno, Reference Sawada and Kaneno1992).
Our inventory of the shrew cestode collection from Sakhalin revealed 33 species of 19 genera of tapeworms (Zubova et al., Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008a) (Table 2). Spalania crassisaccata (=Ecrinolepis crassisaccata) was incorrectly identified earlier as S. сollaris Karpenko, Reference Karpenko and Cherepanov1984 (=Ecrinolepis collaris), a species parasitizing shrews in Siberia and the Russian Far East (Karpenko, Reference Karpenko and Cherepanov1984, Reference Karpenko2004; Odnokurtsev & Karpenko, Reference Odnokurtsev and Karpenko1993; Melnikova et al., Reference Melnikova, Gulyaev, Dokuchaev and Alekseev2003, Reference Melnikova, Gulyaev and Dokuchaev2005; Kornienko et al., Reference Kornienko, Makarikov and Dokuchaev2014, Reference Kornienko, Dokuchaev and Odnokurtsev2018; Kornienko & Dokuchaev, Reference Kornienko and Dokuchaev2023). On the studied islands, it had been replaced by the morphologically similar S. crassisaccata. Specimens previously identified as Neoskrjabinolepis formosa by Zubova et al., (Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008a) were recognised by the present study as belonging to N. kedrovensis. The cestode genus that had the greatest number of species on Sakhalin was Neoskrjabinolepis (six species). The genera Lineolepis, Soricinia, and Staphylocystis were represented by three species each, and the genera Ecrinolepis, Mathevolepis, Novobrachylepis, and Spalania by two species each. The remaining genera were represented by one species each. Specimens of Staphylocystis and Lineolepis differed from the known species of these genera and were recognised as new species.
In the north of the Sakhalin Island, only three S. caecutiens individuals were caught, in which four species of cestodes were found (Table 2). In the southern and central parts of the island, 32 and 28 species of cestodes were found, respectively, and some differences in species composition were noted. These differences most likely were due to sample sizes from different parts of the island (259 specimens in the south and 54 specimens in the central part of the island). In the south of the island, E. longibursata was not found. Given that E. longibursata was recorded on central Sakhalin and Hokkaido, it can be supposed that this species might be present on southern Sakhalin, too.
Previously, we had found 18 species of cestodes of 14 genera in shrews in Hokkaido (Zubova et al., Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Movsessian, Be′er, Zinovieva, Pelgunov and Spiridonov2008b). The cestode genus having the greatest number of taxa on Hokkaido was Neoskrjabinolepis (four species) (Table 2). The genera Ecrinolepis, Lineolepis, Skrjabinacanthus, and Staphylocystis proved to be represented by two taxa each, whereas Mathevolepis, Novobrachylepis, and Soricinia were registered by one species each. Representatives of the genera Spasskylepis and Staphylocystoides were not found in Hokkaido. The analysis of some previous articles (Sato et al., Reference Sato, Kamiya and Ohbayashi1988; Sawada & Koyasu, Reference Sawada and Koyasu1991, Reference Sawada and Koyasu1995; Sawada & Asakawa, Reference Sawada and Asakawa1992; Sawada & Kaneno, Reference Sawada and Kaneno1992; Sawada & Kobayashi, Reference Sawada and Kobayashi1993) containing clear-cut photographs of cestodes allowed the expansion of the list of cestode species parasitizing shrews in Hokkaido to 23 taxa (Table 2). The number of cestode species on southwestern Hokkaido was almost 25% less than on central Hokkaido (14 and 19 species, respectively) (Table 2). The data published by Japanese parasitologists (Sato et al., Reference Sato, Kamiya and Ohbayashi1988; Sawada & Koyasu, Reference Sawada and Koyasu1991; Sawada & Asakawa, Reference Sawada and Asakawa1992; Sawada & Kaneno, Reference Sawada and Kaneno1992) enabled us to estimate the species diversity of cestodes in the north and east of the island, where 12 and 9 species, respectively, were recorded (Table 2).
Shrews of Kunashir were parasitized by 22 cestode species of 14 genera (Table 2). The collection contained larvae of Mesocestoides sp., which had not been mentioned before (Karpenko, Reference Karpenko and Fedorov1997; Kornienko et al., Reference Kornienko, Zubova, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008b). The most diverse cestode genera on Kunashir were Neoskrjabinolepis and Staphylocystis (four and three species, respectively). Most of cestode genera (Lineolepis, Mathevolepis, Monocercus, Novobrachylepis, and Spalania) were represented by one taxon (Table 2). Cestodes of the genera Ecrinolepis, Spasskylepis, and Staphylocystoides were not found on the island.
Of the 36 species of the cestode complex of shrews from Sakhalin–Hokkaido–Kunashir, fewer than half (15) of 13 genera were shared by all three islands (Table 2). On Sakhalin, the number of cestode species (33) was one-third greater than that on Kunashir and Hokkaido (22 and 23, respectively); this was due to the finding on Sakhalin of such mainland species as E. macrospina, M. larbicus, N. sorextscherskii, S. tiunovi, and S. spasskii. All the species parasitizing shrews of Kunashir were also registered on Sakhalin and amounted to 67% of the species diversity of Sakhalin. Between Hokkaido and Sakhalin, there were 20 common species (56%). Although the same number of cestode species had been recorded in Hokkaido and Kunashir shrews, only 17 species (61%) were shared between them.
A comparative analysis of species composition of shrew cestodes between the mainland (Primorsky Krai and Khabarovsk Krai) and the islands showed that 48 species of cestodes of 19 genera were registered on island and mainland; of them, one third (35%) were ubiquitous here (Karpenko, Reference Karpenko and Fedorov1997; Melnikova et al., Reference Melnikova, Gulyaev, Dokuchaev and Alekseev2003, Reference Melnikova, Gulyaev and Dokuchaev2005; Kornienko et al., Reference Kornienko, Zubova, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008b; Zubova et al., Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008a, Reference Zubova, Kornienko, Gulyaev, Dokuchaev, Movsessian, Be′er, Zinovieva, Pelgunov and Spiridonovb). Despite similar numbers of cestode species recorded in shrews on the mainland and on Sakhalin Island (35 and 33 species, respectively), these regions differed appreciably in taxonomic composition: fewer than half (23 taxa) were common between the mainland and Sakhalin (Table 2). Jaccard similarity coefficients for species complexes of shrew cestodes on the considered territories (Sakhalin versus mainland 0.51, Sakhalin vs. Hokkaido 0.56, Sakhalin vs. Kunashir 0.67, and Kunashir vs. Hokkaido 0.61) indicated comparable levels of similarity between the mainland and islands Sakhalin, Hokkaido, and Kunashir.
We performed a comparative analysis of the distribution of cestodes among three species of shrews (S. caecutiens, S. gracillimus, and S. unguiculatus) among the previously mentioned islands and found considerable differences. Insignificant sampling of the remaining shrew species made it impossible to include them in the analysis (Table 1). On the studied islands, the largest numbers of cestode species were recorded in S. unguiculatus (32) and S. caecutiens (29). In S. gracillimus, 19 cestode taxa were found. S. unguiculatus had the highest species diversity of cestodes on all islands (Table 3).
Table 3. Distribution of cestodes among shrew species (Sorex) on islands Sakhalin, Kunashir and Hokkaido (Sc: Sorex caecutiens; Sg: S. gracillimus; Sm: S. minutissimus; Su: S. unguiculatus)

* Shrew cestodes of Kunashir Island from Karpenko’s collection (1997).
** Species of shrew cestodes from Hokkaido Island that have been found by Japanese authors (Sato et al., Reference Sato, Kamiya and Ohbayashi1988; Sawada & Koyasu, Reference Sawada and Koyasu1991, Reference Sawada and Koyasu1995; Sawada & Asakawa, Reference Sawada and Asakawa1992; Sawada & Kaneno, Reference Sawada and Kaneno1992).
On Sakhalin, S. unguiculatus and S. caecutiens were infected by similar numbers of cestode species (30 and 26, respectively). Only 17 species were found in S. gracillimus. Fewer than half of the species of cestodes (13 species) used as definitive host all three species of soricids (Table 3). S. unguiculatus on Hokkaido and Kunashir were parasitized by the largest number (20) of cestode species. On Hokkaido in S. caecutiens and S. gracillimus, 10 and seven cestode species, respectively, were found. On Kunashir Island, S. gracillimus was a definitive host for 12 species, whereas S. caecutiens had only two (Table 3).
Most species of cestodes parasitized all three species of shrews, whereas a few cestodes species were found only in a single shrew species. N. nana was found only in S. gracillimus and the larval stages of D. undula and Mesocestoides sp. were detected only in S. unguiculatus (Table 3). On Hokkaido, only cestodes N. kunashiriensis, N. nuda, and S. diplocoronathus parasitized all three shrew species; one half of the species of cestodes was detected in S. unguiculatus alone. Cestodes D. ezoensis and S. jakutensis were found only in S. gracillimus, and the endemic E. ezoensis occurred only in S. caecutiens (Table 3). On Kunashir, only N. nadtochijae and N. morosovi were shared by all species of shrews and nine out of 20 cestode species were found in S. unguiculatus alone (Table 3).
Discussion
Data on species diversity of shrew cestodes on Sakhalin Island had remained very scarce until the beginning of this century. At the time, there was only one publication (Sawada & Kobayashi, Reference Sawada and Kobayashi1993) on cestodes of soricid mammals of Sakhalin; that paper showed only three species of cestodes: Ditestolepis longicirrosa Sawada & Harada, Reference Sawada and Harada1990, Neoskrjabinolepis singularis (Cholodkovsky, 1912) Spassky, Reference Spassky1954 and Soricinia japonica Sawada & Koyasu, Reference Sawada and Koyasu1991. Our analysis of the drawings and descriptions of cestodes from that publication allowed us to clarify their taxonomic position. The photographs of D. longicirrosa presented by Sawada and Kobayashi (Reference Sawada and Kobayashi1993) as well as in the original description of the species (Sawada & Harada, Reference Sawada and Harada1990) actually match Ecrinolepis longibursata.
At the time of publication by Sawada and Kobayashi (Reference Sawada and Kobayashi1993), there was a longstanding discussion about the existence within the genus Neoskrjabinolepis of one or two species having a trans-Palearctic range (i.e., N. singularis and N. schaldybini Spassky, 1947 [Kornienko et al., Reference Kornienko, Makarikova and Dokuchaev2023]). Later, we proved the validity of both species, and a further 15 species have been described within the genus (Kornienko et al., Reference Kornienko, Gulyaev and Melnikova2006, Reference Kornienko, Makarikova and Dokuchaev2023). Additionally, it has been reported that N. singularis does not occur east of Transbaikalia (Kornienko et al., Reference Kornienko, Makarikova and Dokuchaev2023). Photos of rostellar hooks of N. singularis and their size reported by Sawada and Kobayashi (Reference Sawada and Kobayashi1993) correspond to N. kedrovensis.
The original description of Soricinia japonica by Sawada & Koyasu (Reference Sawada and Koyasu1991) was based on fragments of strobila of two species of the tribe Ditestolepidini, including a species with serial metamerism and a species with gradual metamerism; this led to an incorrect species diagnosis and to and assumption that this was a new species. Two photographs of the general view of the cestode presented by Sawada & Koyasu (Reference Sawada and Koyasu1991) clearly illustrated the difference in the structure of the strobila. The left photo there showed a cestode with a gradual strobilar maturation corresponding to Novobrachylepis morosovi, and the right photo presented a strobila with serial maturation corresponding to Mathevolepis skrjabini. Therefore, Sawada and Kobayashi (Reference Sawada and Kobayashi1993) recorded four species of cestodes on Sakhalin: E. longibursata, M. skrjabini, N. kedrovensis, and N. morosovi. Later, several new cestode species have been described from Sakhalin shrews belonging to genera Soricinia, Spasskylepis, and Neoskrjabinolepis: S. sawadai, S. tiunovi, N. nana, and N. paradoxa (Lykova et al., Reference Lykova, Melnikova and Karpenko2005; Zubova et al., Reference Zubova, Gulyaev and Kornienko2010; Kornienko & Gulyaev, Reference Kornienko and Gulyaev2011; Kornienko et al., Reference Kornienko, Makarikova and Dokuchaev2023).
Until now, only two papers have been published presenting information about helminth fauna of soricid mammals on Kunashir (Karpenko, Reference Karpenko and Fedorov1997; Kornienko et al., Reference Kornienko, Zubova, Gulyaev, Dokuchaev, Galaktionov and Dobrovolskij2008b). N. singularis and L. scutigera are among the 11 species of cestodes found by Karpenko (Reference Karpenko and Fedorov1997) (Table 2, indicated with an asterisk). N. kunashiriensis, N. nadtochijae, and N. nuda have erroneously been reported under the name N. singularis. The cestodes earlier labelled as L. scutigera were identified by us as L. parva. Besides, we found a species of Staphylocystis, which is identical to a cestode from shrews of Sakhalin, which further studies may find to represent a new species.
In shrews from Hokkaido Island, 18 cestode species have been detected (11 of them described as new taxa). These are Choanotaenia sp. Sawada & Koyasu, Reference Sawada and Koyasu1991, Coronacanthus parvihamatus Sawada & Koyasu, Reference Sawada and Koyasu1990, Ditestolepis cyclocephala Sawada & Koyasu, Reference Sawada and Koyasu1991, D. longicirrosa Sawada & Harada, Reference Sawada and Harada1990, D. ezoensis Sawada & Koyasu, Reference Sawada and Koyasu1991, D. crassisaccata Sawada & Asakawa, Reference Sawada and Asakawa1992, Hymenolepis magnirostellata Sawada & Kaneno, Reference Sawada and Kaneno1992; Insectivorolepis macracetabulosa Sawada & Koyasu, Reference Sawada and Koyasu1991, Sinuterilepis ezoensis Sawada & Koyasu, Reference Sawada and Koyasu1995, Soricinia japonica Sawada & Koyasu, Reference Sawada and Koyasu1991, Vampirolepis sp. Sawada & Asakawa, Reference Sawada and Asakawa1992 (Sato et al., Reference Sato, Kamiya and Ohbayashi1988; Sawada & Koyasu, Reference Sawada and Koyasu1991, Reference Sawada and Koyasu1995; Sawada & Asakawa, Reference Sawada and Asakawa1992; Sawada & Kaneno, Reference Sawada and Kaneno1992; Sawada, Reference Sawada1999). As a consequence, a belief has emerged about the high species diversity and high endemism of shrew cestodes on Hokkaido Island. Descriptions of some of these species have been superficial, with many inaccuracies, and have been accompanied by schematic drawings of segments or blurry photographs. Some of these articles are brief faunistic summaries of discovered cestodes, without reporting their morphological features. Several species were misidentified and reported by the same species name. Nonetheless, descriptions and photographs of several species presented by the above authors allowed to clarify the taxonomic position of several species. The cestode Ditestolepis longicirrosa described by Sawada and Harada (Reference Sawada and Harada1990) matches the species Ecrinolepis longibursata. The species Coronacanthus parvihamatus described by Sawada and Koyasu (Reference Sawada and Koyasu1990), is actually U. prolifer. In the next year, Sawada and Koyasu (Reference Sawada and Koyasu1991) described several further new species: Ditestolepis ezoensis, Insectivorolepis macracetabulosa, and Soricinia japonica. Later, D. ezoensis was found in shrews of Sakhalin, redescribed and transferred to the genus Diorchilepis erected for it (Lykova et al., Reference Lykova, Gulyaev, Melnikova and Karpenko2006). I. macracetabulosa has been recognised as a synonym of Soricinia quarta (Zubova et al., Reference Zubova, Gulyaev and Kornienko2010).
Sawada and Koyasu (Reference Sawada and Koyasu1991) found a cestode of the genus Choanotaenia at the juvenile stage of development. The absence of morphological characteristics (except for the number and size of rostellar hooks and other features) did not permit to identify this cestode. Currently, the cestode species from soricid hosts originally designated as Choanotaenia spp. are considered members of the genus Monocercus Villot, 1882 (Melnikova & Gulyaev, Reference Melnikova and Gulyaev2004).
Sawada and Kaneno (Reference Sawada and Kaneno1992) provided a description and photographs of a new species, Hymenolepis magnirostellata, with the shape of the scolex and extended unarmed rostellum matching the genus Staphylocystis. Most likely, its rostellar hooks were lost because of maceration of the strobila. The quality of the description does not allow determining taxonomic position of this cestode.
Sawada and Asakawa (Reference Sawada and Asakawa1992) described another new species, Ditestolepis crassisaccata, which has later been transferred to the genus Spalania Karpenko, Reference Karpenko, Volkov and Marchenko1998, and redescribed as S. crassisaccata (Sawada & Asakawa, Reference Sawada and Asakawa1992) Karpenko, Reference Karpenko, Volkov and Marchenko1998 (Karpenko, Reference Karpenko, Volkov and Marchenko1998; Karpenko & Chechulin, Reference Karpenko and Chechulin2000).
Sawada and Asakawa (Reference Sawada and Asakawa1992) reported a juvenile specimen of Vampirolepis Spassky, Reference Spassky1954 as Vampirolepis sp. Vaucher (Reference Vaucher1992) revised the genus Vampirolepis, all hymenolepidid cestodes parasitizing bats and having armed rostellar apparatus have been transferred to it. Cestodes from soricids with similar structure of the rostellar apparatus and rostellar hooks were placed in the genus Staphylocystis (Spassky, Reference Spassky1954; Сzaplinski & Vaucher, 1994). Therefore, the cestodes Vampirolepis sp. and H. magnirostellata found in shrews of Hokkaido belong to the genus Staphylocystis. Because their species identification remains questionable, we designated shrew cestodes from Hokkaido as Staphylocystis sp. (Tables 2 and 3). Sinuterilepis ezoensis, described by Sawada and Koyasu (Reference Sawada and Koyasu1995), has been transferred to the genus Ecrinolepis as Ecrinolepis ezoensis (Gulyaev et al., Reference Gulyaev, Zubova, Dokuchaev and Ohdachi2009).
In three shrew species from Hokkaido, Sato et al. (Reference Sato, Kamiya and Ohbayashi1988) registered 10 species of cestodes, including three species of Neoskrjabinolepis: N. schaldybini, N. singularis, and Neoskrjabinolepis sp. Their drawings of the rostellar hooks match N. kedrovensis, N. kunashiriensis, and N. nuda. Other recorded species were L. skrjabini, S. furcatа, S. diplocoronathus, S. jakutensis, U. prolifer [Syn. Pseudodiorchis prolifer (Villot, 1880) Kisielewska, 1960], L. scutigera (Dujardin, 1845) [Syn. Staphylocystis toxometra (Baer, 1932) Yamaguti, 1959], and M. baicalensis [Syn. Molluscotaenia baicalensis (Eltyshev, 1975)]. As mentioned, L. scutigera does not parasitize shrews of the Pacific Islands; consequently, we believe that those authors found L. parva.
The analysis of the previously mentioned papers and the examination of the collection of shrew cestodes from Sakhalin, Kunashir, and Hokkaido enabled us to improve the list of shrew cestodes in the study area. This group now includes 36 species.
According to the theory of island biogeography, the number of species on islands is smaller than on the continent and depends on many factors. These are the size of islands, their remoteness from the mainland, biotopic conditions of species, species diversity and abundance of hosts, type of helminth life cycle, etc. (MacArthur & Wilson, Reference MacArthur and Wilson1967; Mas-Coma & Feliu, Reference Mas-Coma, Feliu, Kuhbier, Alcover and Guerau d’Arellano1984; Miquel et al., Reference Miquel, Fons, Feliu, Marchand, Torres and Clara1996; Goüy de Bellocq et al., Reference Goüy de Bellocq, Sarà, Casanova, Feliu and Morand2003; Bugmyrin, Reference Bugmyrin2014). The results of a comparative analysis of the cestode diversity of shrews from the mainland and the Sakhalin – Kunashir – Hokkaido island complex are contradictory and do not provide an unambiguous answer.
Although there are fewer species of shrews (6) on Sakhalin than on the mainland (9), they are parasitized by almost the same number of cestode species (35 and 33, respectively). This can most likely be explained by the short distance between Sakhalin and the mainland, the similarity of their current physical-geographical state, similar feeding spectrum, broad host specificity of cestodes and the history of the island’s formation.
According to the theory of island biogeography, the number of species on an island is proportional to its size and distance from the mainland. The diversity of shrew cestodes on Hokkaido is one third less (23 species) than on Sakhalin, given their relatively similar area. This is probably due to the remoteness of Hokkaido from the mainland as well as the history of the island’s formation. During the Pleistocene, the islands were repeatedly separated from the mainland. It is known that the last separation of Sakhalin from Hokkaido occurred approximately 12,000 years ago, and the last separation of Sakhalin from mainland was 7000 years ago (Velizhanin, Reference Velizhanin1976). This resulted into a relatively brief isolation, both among the islands and between those islands and the mainland, which allowed some cestode species to penetrate from the mainland to Sakhalin. Kunashir Island has c. 50 times smaller size than Sakhalin and Hokkaido. However, almost the same number of cestode species (22 species) as in Hokkaido was detected. Probably, the similarity of habitats on Hokkaido and Kunashir and their recent separation (75,000 years ago) formed the basis of the species richness of cestodes.
We have not found a direct connection between helminth species diversity and host species’ diversity and host abundance. The cestodes of shrews are characterised by broad host specificity. The same cestode species can be found in different species of Sorex (e.g., S. diplocoronathus has been found in all shrew species on the islands), although individual cestode species may parasitize only one host species (e.g., N. nana has been found in S. gracillimus only). This is most likely due to the similar food spectrum of different species of shrews. The absence of certain invertebrates (intermediate hosts of cestodes) needed for the realization of the cestode life cycle may lead to the extinction of a number of cestode species on the islands.
The highest number of cestode species on the studied islands was recorded for S. unguiculatus. Species richness of cestodes in S. unguiculatus on Hokkaido and Kunashir is one-third less than this on Sakhalin. In S. caecutiens from Hokkaido Island, almost three times fewer species of cestodes were registered as compared to Sakhalin (10 and 26, respectively). The lowest number of cestode species on all studied islands was recorded in S. gracillimus. S. unguiculatus was dominant in the shrew community on the studied islands (Voronov et al., Reference Voronov, Zagorodskih and Perminov1969; Nesterenko, Reference Nesterenko1999; Loktionova et al., Reference Loktionova, Nesterenko and Burkovsky2016; Nesterenko et al., Reference Nesterenko, Loktionova and Burkovsky2016). As a rule, the dominant position of a certain shrew species in the community of various shrews ensures its leading role in the maintenance of cestode infection in shrew communities (Kornienko et al., Reference Kornienko, Makarikov and Dokuchaev2014; Kornienko & Dokuchaev, Reference Kornienko and Dokuchaev2023).
On the other hand, we found almost the same number of cestode species in S. unguiculatus and S. caecutiens (30 and 26 species, respectively) on Sakhalin, although the sample size of the two shrew species differed by several times. A similar situation was found for S. unguiculatus in Hokkaido and Kunashir. At the same time, it is undeniable that the chance of finding more cestode species increases with increasing host abundance. Species richness of shrew cestodes on the islands could increase if host specimens consisted of at least 40–50 individuals (Poulin, Reference Poulin1998). The species E. longibursata, recorded on the mainland, Sakhalin and Hokkaido, was not found on Kunashir. It is possible that this species also parasitized shrews of Kunashir, and its absence may be explained either by small sample size or by rarity of this cestode species on Kunashir.
It should be noted that, despite the same number of cestode species on the mainland, Sakhalin, Hokkaido and Kunashir, the taxonomic composition of the cestode community varied (Cj=0.51–0.67) (i.e., the cestode genera were represented by different species on the mainland and the different islands).
In the “Sakhalin–Kunashir–Hokkaido” complex of shrew cestodes, mostly eastern Palearctic (16) species were found. Trans-Palearctic taxa are represented by six species (D. diaphana, P. mathevossianae, S. diplocoronathus, S. furcata, S. quarta, and U. prolifer). According to recent data, D. diaphana, which used to be considered a single species, is actually a species complex (Kornienko et al., Reference Kornienko, Binkienė, Dokuchaev and Tkach2019). Therefore, to determine the species identity of D. diaphana sensu lato found on the islands, additional investigations are necessary. Furthermore, according to Spassky and Andrejko (Reference Spassky and Andrejko1970), the name S. furcata harbors several morphologically and ecologically similar species. This notion has been confirmed by our discovery (on islands Sakhalin, Kunashir, and Hokkaido) of cestodes assigned to the genus Staphylocystis but having characteristics different from those of S. furcata. Additional research is needed to determine taxonomic status of the found cestodes.
It has been demonstrated that the biota of Sakhalin and Kuril Islands (Kunashir) features low endemism (Bogatov et al., Reference Bogatov, Pietsch, Zhuravlev, Storozhenko, Lelej, Barkalov, Kholin and Prozorova2003, Reference Bogatov, Pietsch, Storozhenko, Barkalov, Lelej, Kholin, Krestov, Kostenko, Makarchenko, Prozorova and Shedko2006; Pietsch et al., Reference Pietsch, Bogatov, Amaoka, Zhuravlev, Barkalov, Gage, Takahashi, Lele, Storozhenko and Minakawa2003). For example, in the micromammal fauna of Sakhalin, the proportion of endemics is no more than 14%, whereas in the theriofauna of Japan as a whole (including Honshu and other islands), there are ~40% of endemic forms of mammals (Millien-Parra & Jaeger, Reference Millien-Parra and Jaeger1999). Among shrew cestodes of Sakhalin, only two species (N. paradoxа, and S. sawadai) of 33 (6%) are endemic, whereas the cestode fauna of Hokkaido contains one endemic species (E. ezoensis). On Kunashir Island, there are no endemic species of shrew cestodes. Our comparison indicates that approximately 22% (8 of 36 species) of cestodes (D. ezoensis, E. ezoensis, N. kunashiriensis, N. nana, N. nuda, N. paradoxа, S. crassisaccata, and S. sawadai) occur only on the Sakhalin–Kunashir–Hokkaido Island complex and hence are island endemics. For three species of the genera Staphylocystis, Lineolepis and Monocercus, it is needed to further clarify their taxonomic status, which may result in recognizing them as endemics of this island complex.
The presence of trans- and eastern-Palearctic species in the studied faunal complex and the absence of island autochthons among shrews point to repeated and unidirectional incursion of shrews into the islands (primarily on Sakhalin Island) in the Quaternary. According to the “rule of six” (Dokuchaev, Reference Dokuchaev2005), the return migration of shrews was prevented by complete saturation of their communities on the mainland. Multiple invasions from the mainland to the islands at different time points, the complex dynamic structure of the shrew community on the islands depending on their physiographic conditions, the abundance of definitive hosts and the presence of intermediate hosts of cestodes have ultimately caused the high diversity and endemism of shrew cestodes on the studied islands.
Acknowledgements
We thank V.D. Gulyaev, Yu.A. Melnikova, T.A. Makarikova, and A.A. Makarikov for help with the fieldwork in Sakhalin Oblast. We are also very grateful to S.D. Ohdachi for organization of N.E. Dokuchaev’s field work in Hokkaido. The study was supported by Federal Fundamental Scientific Research Programs (No. 1021051703269-9-1.6.12 and 123032000021-4). The English language was corrected and certified by shevchuk-editing.com.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interest
The authors declare no conflict of interest.
Ethical standards
The authors carefully reviewed the ethical standards of the journal and hereby certify that the procedures used with the investigated species comply fully with those standards. The authors assert that all procedures and methods contributing to this work and used in the current study comply with the ethical standards of the relevant national and institutional guides with laws of the Russian Federation and were approved by the ethics committee of the ISEA, Novosibirsk, Russia.






