Introduction
Characteristics of hosts and parasites combine in shaping their relationships over evolutionary time. In this sense, different factors of host biology, such as body size, diet preferences and behaviour, may contribute to or limit encounters with parasites (Poulin, Reference Poulin2007). Several studies have shown the effects of host traits and habitat in the richness, composition and abundance of anuran parasites. In this sense, host body size has often been shown to determine parasite richness (Campião et al., Reference Campião, Ribas, Morais, da Silva and Tavares2015a) and parasite abundance among host individuals for many anuran species (Hamann et al., Reference Hamann, Kehr and González2014; Campião et al., Reference Campião, Ribas and Tavares2015b, Reference Campião, Dias, da Silva, Ferreira and Tavares2016; Toledo et al., Reference Toledo, Schwartz, Nomura, Aguiar, Velota, da Silva and Anjos2017). Nonetheless, some recent studies have reported no evidence between host size and infracommunity structure (Alcantara et al., Reference Alcantara, Ferreira-Silva, Silva, Lins, Ávila, Morais and da Silva2018; González et al., Reference González, Schaefer and Duré2020; Madelaire et al., Reference Madelaire, Franceschini, Morais, Gomes and da Silva2020), since other host characteristics can also affect this relationship (Hamann et al., Reference Hamann, Kehr and González2014; Campião et al., Reference Campião, Ribas, Silva, Dalazen and Tavares2017; Toledo et al., Reference Toledo, Schwartz, Nomura, Aguiar, Velota, da Silva and Anjos2017; Madelaire et al., Reference Madelaire, Franceschini, Morais, Gomes and da Silva2020). For instance, habitat condition is a crucial driver of parasite community structure since transmission and infection processes are directly related to changes in the environment (Thieltges et al., Reference Thieltges, Jensen and Poulin2008). Moreover, environmental changes may be associated with disease emergence and increased infections in anurans (Blaustein & Johnson, Reference Blaustein and Johnson2003; Beasley et al., Reference Beasley, Faeh, Wikoff and Lannoo2005; Johnson et al., Reference Johnson, Calhoun, Riepe, McDevitt-Galles and Koprivnikar2019). Nonetheless, parasite communities, in general, tend to reflect ecosystem heterogeneity and complexity, and are negatively affected by environmental alterations (King et al., Reference King, Mclaughlin, Boily and Marcogliese2010; Schotthoefer et al., Reference Schotthoefer, Rohr, Cole, Koehler, Johnson, Johnson and Beasley2011; Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012; Campião et al., Reference Campião, Ribas, Silva, Dalazen and Tavares2017), which points to the challenge in predicting such patterns among host individuals.
Understanding the mechanisms structuring host–parasite interactions is a fundamental matter in parasitology. The hierarchical nature of parasite assemblages within host individuals, populations and communities provide a good baseline to address this issue (Poulin, Reference Poulin2007). Different host and parasite species can interact with each other and generate complex ecological networks of interactions, in which species are nodes connected to each other by links that represent their interactions (Poulin, Reference Poulin2010; Godfrey, Reference Godfrey2013). Traditionally, most studies have described the non-random organization of parasite–host networks and how they vary through space-time by considering this relationship at the species level. In such interspecific interaction networks, all records of the interactions of individuals of a species observed in the field are pooled together and represented as a single node in the network, ignoring the intra-population variation of ecological interactions (Benitez-Malvido et al., Reference Benitez-Malvido, Martínez-Falcón, Dattilo, González-DiPierro, Estrada and Traveset2016). However, the individual is a truly discrete study system and the actual unit through which natural selection operates. In this context, since a single host individual can be associated with several parasite species in a predictable way, a network approach can also be used to evaluate the structure of how host–parasite relationships vary among individuals of the same population (Godfrey et al., Reference Godfrey, Bull, James and Murray2009; Fenner et al., Reference Fenner, Godfrey and Bull2011). In these individual-based networks, parasite species and host individuals are depicted as nodes, and their relationships by links describing the presence of parasite species in host individuals. Despite the increasing number of studies on host–parasite networks at the species level, there is still little knowledge about how and why individual-based host–parasite networks are structured and vary across ecological gradients.
In this study, we used network analysis to describe intra-populational variation in relationships involving five anuran species and their parasite communities under contrasting environmental conditions (i.e. a pasture and a natural reserve site) in south-eastern Pantanal, Brazil. Here, we aimed to address whether the parasite community composition and the structure of individual-based networks of anuran species and their parasites vary between two study sites. Specifically, we tested for the following differences between the two sites: (1) the variance in the beta diversity of parasite communities; (2) the structure (i.e. parasite richness, interaction diversity, specialization, nestedness and modularity) of individual-based anuran–parasite networks; and (3) the contribution of individual host traits (i.e. body size) to their interaction strength within the networks.
Materials and methods
Study area and data collection
Data were collected in south-eastern Pantanal, Brazil, where the average monthly temperature ranges between 23°C and 28°C, with a rainy season from October to April and a dry season from May to September. The Pantanal wetland presents an impressive landscape mosaic, which, in combination with the warm temperature, contributes to great anuran abundance. Anurans were collected in ponds in farmland that harbours vast grasslands and natural fields for extensive livestock farming, and in a pond in a forested protected area consisting of a legal natural reserve (600 ha) that has no cattle farming. The two study sites have contrasting characteristics. The pond in the pasture area (19°03.397'S 56°47.011'W) is in an open field, has high exposure to solar radiation and is eutrophic due to excessive organic matter deposition from the accumulation of cattle excreta (0.27 mg/l of phosphorus and 9.5 mg/l of nitrogen). On the other hand, the pond in the natural reserve (19°03.885'S, 56°45.000'W) is surrounded by forest and receives lower solar radiation and organic matter than the ponds in the pasture area (phosphorus as 0.11 mg/l and nitrogen as 3.5 mg/l) (Campião et al., Reference Campião, Ribas, Silva, Dalazen and Tavares2017).
Collections occurred in both sites between January and February 2011 and in December 2013. Five anuran species with different life histories and of two different families were surveyed: the arboreal Pithecopus azureus (=Phyllomedusa azurea; 17 in the pasture site and 12 in the natural reserve) and the semi-aquatic Pseudis paradoxa (20 in the pasture site and 17 in the natural reserve), both of the family Hylidae; and Leptodactylus chaquensis (ten in the pasture site and ten in the natural reserve), Leptodactylus fuscus (19 in the pasture site and 11 in the natural reserve) and Leptodactylus podicipinus (22 in the pasture site and 13 in the natural reserve), which live in the interface between aquatic and terrestrial habitats and belong to the Leptodactylidae. Despite some anuran species being collected in different sampling periods, each species was collected in the same field trip in both environments, which may help to avoid potential biases in data analyses. Anurans were euthanatized with an overdose of sodium thiopental solution (0.01 ml/g injected into the body cavity) and had all their organs examined for parasites. Most parasite taxa were identified to species level, but when this was not possible, they were distinguished as morphotypes according to morphological characteristics. Details on the collection of host–parasite data are described in Campião et al. (Reference Campião, Ribas, Silva, Dalazen and Tavares2017). Eight parasite taxa were not identified to species or morphotype level because they were either found as early larval stages or only as females. In both cases, there were a lack of morphological structures for identification and so these parasite taxa were excluded from analyses to avoid confounding effects in the results.
Data analyses
Infracommunity refers to all parasites found in a host individual. We calculated the beta diversity of parasite infracommunities for all anurans in both sites, which we then analysed following a multivariate approach. First, we created a matrix with individual anurans and their associated parasites for each study site, and calculated the similarities in the composition and abundance of these parasite infracommunities with the Jaccard and Sorensen similarity indexes, respectively. Then we measured the dispersion of these indexes for each study site by calculating their average distances to the spatial median (i.e. centroid) in multivariate space with an analysis of multivariate homogeneity of groups dispersions. This analysis is analogue to Levene's inferential statistic for homogeneity of variances among groups (Anderson et al., Reference Anderson, Ellingsen and McArdle2006). We then used a permutation test of multivariate homogeneity of group dispersions to test if either study site is more variable than the other in the distances of its infracommunities to group centroid. In this analysis, we considered only the variation in the beta diversity of the local pool of parasites (i.e. infracommunities) in each study site and did not consider the identity of anuran hosts. In a second analysis, we analysed the variation in the beta diversity of parasite infracommunities considering also host identity (i.e. anuran species). For that, we used the Jaccard and Sorensen similarity indexes and tested if they were related to host species and study site with a linear model.
We used each anuran population and their parasites in each environmental condition (pasture and natural reserve) as independent anuran–parasite networks A, where aij = number of parasite species i recorded on individual anuran j, and zero otherwise, thus totalling ten individual-based networks – one for each of the anuran species in each of the environmental conditions. We categorized parasite species as peripheral (selective species, those with fewer interactions) or central core (generalist species, those with the most interactions) components of the networks according to Dáttilo et al. (Reference Dáttilo, Guimarães and Izzo2013). This categorization enabled us to evaluate spatial turnover in the specific positions of parasite species within each network (e.g. shifting from peripheral to generalist core between the natural reserve and pasture networks).
It is important to note that we used only weighted network descriptors, mainly because recent studies have shown that weighted networks tend to be more robust to sampling bias compared to binary networks (Vizentin-Bugoni et al., Reference Vizentin-Bugoni, Maruyama, Debastiani, Duarte, Dalsgaard and Sazima2016). To estimate interaction diversity (ID), we used a descriptor based on the exponential value of the Shannon index to calculate the number of true or equivalent interactions in each network (Corro et al., Reference Corro, Ahuatzin, Aguirre, Favila, Ribeiro, Acosta and Dáttilo2019). Network specialization was quantified by the H 2' index, which is based on deviation from the expected probability distribution of chance interactions. For this index, extreme generalization of a network is H 2' = 0 and extreme specialization is H 2' = 1 (Blüthgen et al., Reference Blüthgen, Menzel and Blüthgen2006).
We tested whether within each anuran–parasite network there were groups of parasite species strongly associated with a particular set of individual anurans, as expected in a modular individual-based network. For this, we calculated weighted modularity (Q) computed by the QuanBiMo algorithm, which ranges from 0 (no more links within modules than expected by chance) to 1 (maximum possible modularity) (Dormann & Strauß, Reference Dormann and Strauß2014). Moreover, we also estimated nestedness using wNODF (Almeida-Neto & Ulrich, Reference Almeida-Neto and Ulrich2011), which varies from zero (not nested) to 100 (perfectly nested). In this case, we specifically evaluated if selective parasite species would interact with only a subset of anuran individuals that interact with the generalist parasite species (i.e. a nested pattern of anuran–parasite interactions). We generated a random network to test the significance of modularity and nestedness according to the Patefield null model (n = 500), which fixes the network size and the marginal totals while shuffling interactions randomly in the matrix (Dormann et al., Reference Dormann, Gruber and Fruend2008).
The strength of individual anurans and parasites within each network was calculated according to Bascompte & Jordano (Reference Bascompte, Jordano, Pascual and Dunne2006). This metric quantifies the level of dependence of a given species to the assemblage of species in the other trophic level with which it interacts, and, therefore, reflects the importance of each species within the network. A generalized linear model was used to test the relationship among interaction strength, host body size and environmental condition (i.e. pasture and reserve), and paired t-tests for each anuran species were used to test differences in the values of each network descriptor between the pasture and natural reserve.
Statistical analyses were conducted in R software (R Development Core Team, 2019). Community similarities, beta diversity index calculation and multivariate dispersion were calculated with the betapair and betadisper functions, respectively, and the linear models with the aov and glm functions, using the vegan package (Oksanen et al., Reference Oksanen, Blanchet and Friendly2019). Network analyses were conducted using the bipartite package (Dormann et al., Reference Dormann, Gruber and Fruend2008).
Results
Parasite species richness for each anuran population was similar across study sites: six parasite taxa in the pasture site and five in the natural reserve for P. azureus; two parasite taxa in the pasture site and five in the natural reserve for P. paradoxa; nine parasite taxa in the pasture site and eight in the natural reserve for L. chaquensis; seven parasite taxa in the pasture site and six in the natural reserve for L. fuscus; and six parasite taxa for L. podicipinus in both sites. After applying the inclusion criteria, the following 17 parasite morphotypes composed these communities: the nematodes Aplectana sp., Brevimulticaecum sp., Cosmocerca parva, Cosmocerca podicipinus, Cosmocercella phyllomedusae, Physaloptera sp., Physalopteroides venancioi, Physocephalus sp. 1, Physocephalus sp. 2, Physocephalus sp. 3, Porrocaecum sp., Raillietnema minor, Schrankiana formosula and Schrankiana fuscus; and the trematodes Diplostomulum sp., Glypthelmins palmipedis and Neascus sp.
The nematodes C. podicipinus, S. formosula and Brevimulticaecum sp. 1 and the trematode G. palmipedis were the most connected parasite taxa, interacting with the greatest number of host individuals (40, 21, 20 and 19, respectively). Most parasite taxa occurred in both sites, except for C. phyllomedusae and Neascus sp. 1, which were found only in the pasture site, and C. parva and Physocephalus sp. 2, which were found only in the reserve. Thus, considering the local pool of parasites in each site, the variations in the beta diversity of infracommunities did not differ between the pasture and the natural reserve (permutation test for homogeneity of multivariate dispersions of parasite occurrence F = 1.22, df = 1, P = 0.26), and abundance (permutation test for homogeneity of multivariate dispersions of parasite abundance F = 1.77, df = 1, P-value = 0.17), since there was high heterogeneity among infracommunities in both sites (supplementary figs S1 and S2). However, when we analysed the variation in the beta diversity of parasite infracommunities between sites considering host identity, species abundance tends to differ by host species (table 1).
Table 1. Analysis of variance of the beta diversity among parasite communities of five anuran species from contrasting study sites (pasture and natural reserve) fitted as a linear model.

The beta diversity index was calculated considering parasite species composition with the Jaccard similarity index, and parasite species abundance considering the Sorensen similarity index.
Despite the overall differences in the communities, the composition of core species of most networks remained the same in both sites (C. podicipinus was a core species in both sites for L. podicipinus, in the pasture site for L. fuscus and the natural reserve for L. chaquensis; G. palmipedis was a core species in both sites for P. paradoxa; and R. minor was a core species in both sites for P. azureus). Evaluating non-random patterns in the networks among hosts and parasites revealed that all networks exhibited a significantly modular structure when compared to neutral patterns of host–parasite interactions (null models) (all P-values < 0.05). This finding indicates that there are groups of parasite species that specifically parasitize particular groups of host individuals. Besides, no network was significantly nested when compared to the neutral patterns of anuran–parasite interactions (null models) (all P-values > 0.05), indicating that the interactions recorded for host individuals scarcely exploited by parasite species were not a cohesive subset of the interactions found on the most parasitized host individuals.
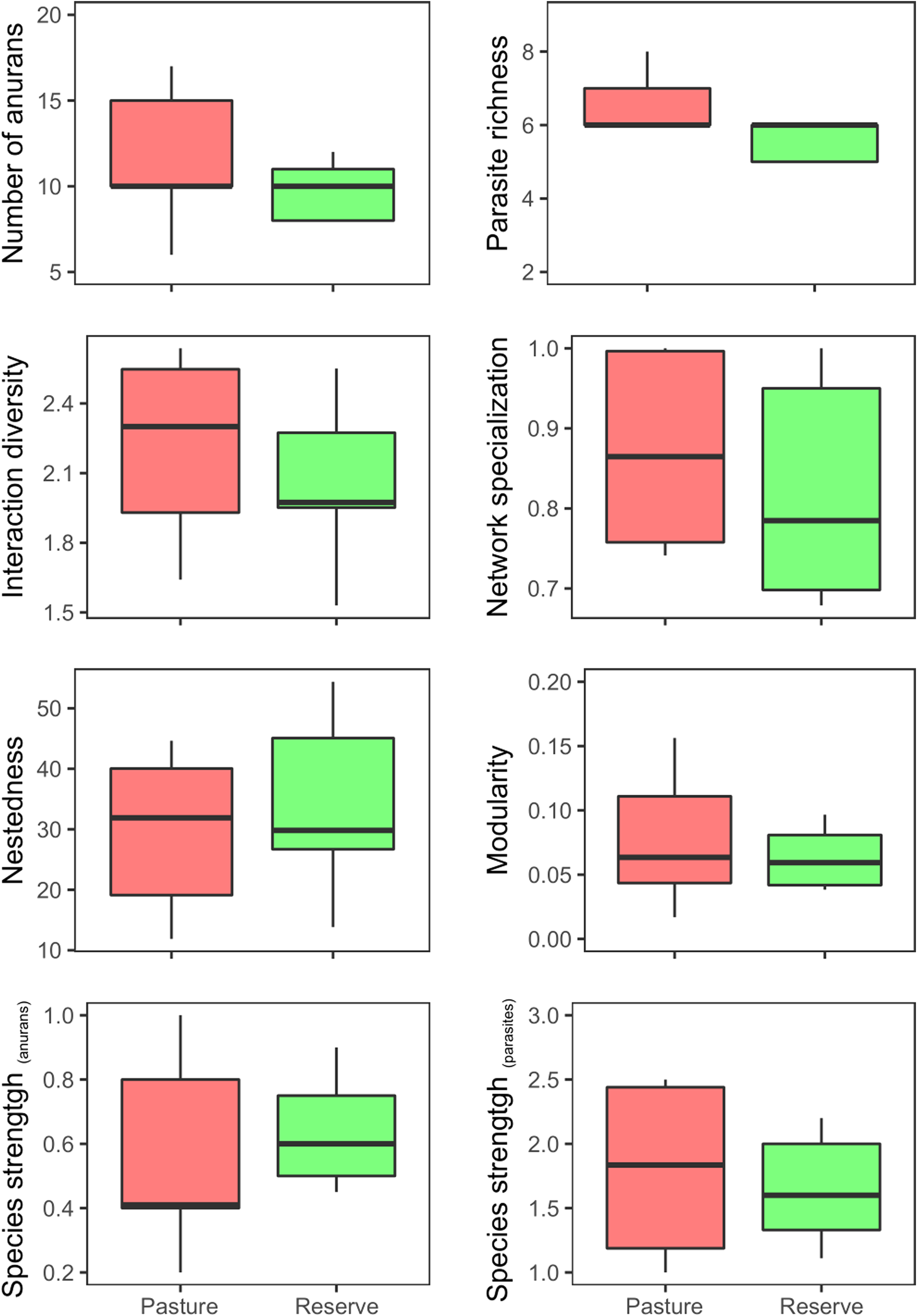
We found that the structure of the individual-based anuran–parasite networks did not change between pasture and natural reserve (fig. 1; supplementary table S1). Moreover, we observed that parasite species had greater interaction strengths than their hosts (t = 3.25, DF = 9, P < 0.0001; supplementary table S2 and fig. S3). Also, we found that host traits were important drivers of network structure (fig. 2), since the interaction strength of host individuals was positively related with their body size (deviance = 35.2, P < 0.001), independent of the study site (i.e. pasture and reserve; deviance = 0.305, P = 0.58).

Fig. 1. Descriptors of the structure of individual-based networks of five anuran species and their parasites under different environmental conditions (i.e. pasture and reserve).

Fig. 2. The relationship between host body size (snout-vent length in mm) and interaction strength in individual-based networks of five anuran species and their parasites collected in contrasting environments. The two images are mirrored and represent the same results, but (a) highlights individuals of different species, while (b) highlights individual anurans under different conditions. The smoothed area represents the linear best-fit model (95% confidence intervals).
Discussion
We found that host species was the main filter for parasite community structure, despite the contrasting study sites. Parasites had greater interaction strength than hosts because they had more interactions, and parasite taxa with a higher proportion of interactions in the networks occurred in both the pasture and the natural reserve, which may have contributed to a similar structure in the networks of both (Guimarães et al., Reference Guimarães, Jordano and Thompson2011). Moreover, traits of host individuals were related to network parameters, with larger hosts having a greater number of interactions. There is a considerable amount of information about the mechanisms that cause variation among communities of free-living organisms, but understanding how their ecological interactions vary over time and space is still poorly understood (Poisot et al., Reference Poisot, Guéeveneux, Fortin, Gravel and Legendre2017). Thus, our results add evidence for a better understanding of host–parasite relationships in changing environments.
Most parasite taxa observed in this study were able to successfully associate with their hosts, despite the differences in environmental conditions between the two study sites. It is expected that parasite assemblages will be influenced by the environment directly and indirectly through changes mediated by host assemblages (Berkhout et al., Reference Berkhout, Borregaard, Brandl, Brändle, Dehling, Hof, Poulin and Thieltges2019). This is because local environmental conditions, such as temperature, salinity and pH, may or may not favour free-living infective stages of parasites (Thieltges et al., Reference Thieltges, Jensen and Poulin2008). Additionally, other factors, such as landscape configuration (e.g. habitat connectivity), shape host assemblages, which, in turn, is a determinant of parasite community structure (Berkhout et al., Reference Berkhout, Borregaard, Brandl, Brändle, Dehling, Hof, Poulin and Thieltges2019). Therefore, studying biotic interactions may be more informative than focusing on species alone, since interactions may respond to environmental variables differently (Poisot et al., Reference Poisot, Guéeveneux, Fortin, Gravel and Legendre2017). The similar structure in parasite communities of the different sites studied here was unexpected and may be a result of the similarity in host communities and the tolerance of the most common parasite taxa.
We know that species within a local pool of parasites can vary in their response to changes in environmental conditions (Krasnov & Poulin, Reference Krasnov, Poulin, Morand and Krasnov2010; Koprivnikar et al., Reference Koprivnikar, Marcogliese, Rohr, Orlofske, Raffel and Johnson2012; Poisot et al., Reference Poisot, Guéeveneux, Fortin, Gravel and Legendre2017). In other words, some species may increase in abundance while others decrease, and this will be reflected in their contributions to community dissimilarity. In this sense, differences in parasite community structure among sites can be shaped by changes in parasite prevalence or abundance. The variability in these parameters could result from both environmental characteristics and changes in species identity as mentioned above, but also seem to be true attributes of parasite species (Krasnov & Poulin, Reference Krasnov, Poulin, Morand and Krasnov2010). Highly prevalent parasite species tend to be abundant, and are also potentially capable of broader dispersal. These highly connected species will be influential to network structure, such as the nematode C. podicipinus in the present study, which was a core species in four of the ten studied networks (see Campião et al., Reference Campião, Ribas, Silva, Dalazen and Tavares2017). In this case, realized interactions would result from the opportunity of contact between hosts and parasites, which is determined by their abundances. Our results point to the influence prevalent and abundant parasites, which were core species, and contributed to the similarity in network structure in both sites.
Observing the drivers of network asymmetry from the perspectives of both parasite and host at the infracommunity level is also informative for elucidating what is shaping emergent patterns. For example, heterogeneous populations can be composed of a set of dissimilar individuals, and variation in interaction strength may, in turn, be related to traits such as individual body size. Our results are in accordance with this assumption, as we observed that anuran body size contributes to its interaction strength within each network. Moreover, such heterogeneity within host populations may contribute to parasite aggregation, which is a commonly observed pattern (see Poulin, Reference Poulin2007).
Due to its relationship with intrinsic biological proprieties, such as species dispersion and abundance, body size is expected to influence network structure, which has been shown to be the case for food webs (Naisbit et al., Reference Naisbit, Rohr, Rossberg, Kehrli and Bersier2012). However, predictive models on how the body size of a species will affect host–parasite networks are still scarce in the literature (Bellay et al., Reference Bellay, Oda, Campião, Yamada, Takemoto, Oliveira, Dáttilo and Rico-Gray2018). Here, we showed that anurans with larger bodies have greater interaction strength within anuran–parasite networks, despite the contrasting environmental conditions of the study sites. These anuran hosts are expected to offer more space and niches for parasites, which increases their probability of being associated with more parasites. In fact, larger anurans with long life spans are exposed to parasite sources for longer periods of time, thus leading to an increase in their accumulated parasite richness, as predicted by the niche breadth hypothesis (Poulin, Reference Poulin2007; Kamiya et al., Reference Kamiya, O'Dwyer, Nakagawa and Poulin2014).
We conclude that the combination of factors related to both parasites and hosts contributed to network structure in the studied sites. The great number of parasite interactions, with the composition of core species being stable in both sites, and host body size were the best descriptors of individual-based host–parasite networks, despite contrasting environmental conditions. There might be a greater sensitivity of species interactions capturing variation that is not apparent when looking at species occurrences only (as pointed out by Poisot et al., Reference Poisot, Guéeveneux, Fortin, Gravel and Legendre2017). The mechanisms shaping these interactions might indeed be ubiquitous and pervasive, and a deeper understanding of them will rely on the study of the nature of each interacting organism. Thus, the description of interaction patterns through network analyses in combination with profound knowledge on species biology may contribute to understanding the processes influencing biological communities at different levels of organization.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X20000504
Acknowledgements
This work is a result of an ecological networks workshop, and the authors are deeply grateful to Domingos de Jesus Rodrigues for providing all logistical support and encorouging scientific debate.
Financial support
KMC is grateful to UFPR and WD to Inecol.
Conflicts of interest
None.
Ethical standards
This work follows all ethical standarts.