Introduction
The northern bobwhite (Colinus virginianus; hereafter bobwhite) is a prized upland game bird across its geographic range in the US that is experiencing a long-term population decline. This decline is likely a result of the continued reduction and fragmentation of its habitat (Brennan, Reference Brennan1991; Hernández et al., Reference Hernández, Brennan, DeMaso, Sands and Wester2013), though declines have continued despite attempts to improve habitat (Brennan et al., Reference Brennan, Hernández, Bryant and Brennan2007; Hernández & Peterson, Reference Hernández, Peterson and Brennan2007).
Interest in helminth parasites as a possible proximate factor in the bobwhite decline has spurred a flurry of recent studies within Texas (see review in Peterson & Fedynich, Reference Peterson, Fedynich and Brennanin press). Some studies have focused on species likely to cause pathology such as the eyeworm (Oxyspirura petrowi; Villarreal et al., Reference Villarreal, Fedynich, Brennan and Rollins2012; Bruno et al., Reference Bruno, Fedynich, Smith-Herron and Rollins2015; Dunham et al., Reference Dunham, Bruno, Almas, Rollins, Fedynich, Presley and Kendall2016, Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017; Henry et al., Reference Henry, Brym, Kalyanasundaram and Kendall2017) and the caecal worm (Aulonocephalus pennula; Henry et al., Reference Henry, Brym, Kalyanasundaram and Kendall2017), whereas other studies have used a community survey approach (Olsen & Fedynich, Reference Olsen and Fedynich2016; Villarreal et al., Reference Villarreal, Bruno, Fedynich, Brennan and Rollins2016; Bruno et al., Reference Bruno, Fedynich, Rollins and Wester2018, Reference Bruno, Rollins, Wester and Fedynich2019). Findings from these studies have shown that geographic location, season, year and variation in precipitation can influence helminth species richness, prevalence and abundance in bobwhites, highlighting the importance of temporal helminth surveys in regions characterized by stochastic environmental conditions.
There has been only one recent helminth survey of bobwhites in South Texas, which occurred the two years immediately prior to our study during the 2012–2013 and 2013–2014 bobwhite hunting seasons (Olsen & Fedynich, Reference Olsen and Fedynich2016). This study examined the helminth community in bobwhites during a severe drought period (NOAA, 2020), representing the decline phase of the host's (i.e. bobwhite) boom-and-bust cycle (see Lusk et al., Reference Lusk, Guthery, Peterson and DeMaso2007 for designation of bobwhite population ‘cycles’ in South Texas). These cycles are regulated by variable rainfall, which impacts habitat quality. Tri et al. (Reference Tri, Sands and Buelow2012) found cumulative rainfall from 1 April through 31 August explained 94% of the amount of variation in bobwhite productivity (i.e. age ratios in the fall hunter harvest) within South Texas. Consequently, there is a need to examine helminth communities temporally over the host boom-and-bust cycle to determine whether community dynamics also vary. The objectives of our study were to determine helminth community structure (prevalence and abundance) and patterns (distribution patterns, diversity, numerical dominance) in bobwhites occurring within South Texas during a wet period and relate our findings to the host boom-and-bust cycle within the region and from distinctly different physiographic environments.
Materials and methods
Study area
The study was conducted in South Texas (fig. 1), which represents approximately 9,676,800 ha (Texas Comptroller, 2020). Because of its importance as one of the last remaining regions with good bobwhite populations, South Texas was designated a Legacy Landscape of National Significance for Northern Bobwhite Conservation by the National Bobwhite Conservation Initiative in 2014 (Brennan, Reference Brennan2014).

Fig. 1. Northern bobwhite (Colinus virginianus) collections during the 2014–2015 and 2015–2016 hunting seasons within South Texas (Gould et al., Reference Gould, Hoffman and Rechenthin1960).
The climate of South Texas ranges from humid subtropical along the Gulf of Mexico to warm semi-arid inland along the western Texas–Mexico border. Annual precipitation ranges from 46–97 cm (TPWD, 2020), with periods of drought followed by excessive rain events and localized flooding (Fulbright & Bryant, Reference Fulbright, Bryant, Forgason, Bryant and Genho2003). The region consists of a mixture of prairie grasslands, live oak (Quercus virginiana) woodlands, honey mesquite (Prosopis glandulosa), prickly pear (Opuntia engelmannii) and acacias (Acacia spp.) (Gould, Reference Gould1962; Smeins et al., Reference Smeins, Diamond, Hanselka and Copeland1992; Hernández et al., Reference Hernández, Perez, Guthery and Brennan2007).
Climate data were obtained from the National Centers for Environmental Information interactive GIS map portal (NOAA, 2020) covering the periods April–September 2012–2015. Palmer Drought Severity Index (PDSI) values were averaged across the South Texas region during each of the four periods to determine their respective PDSI range category (extreme drought: −4.00 and below; severe drought: −3.00 to −3.99; moderate drought: −2.00 to −2.99; mid-range: −1.99 to +1.99; moderately moist: +2.00 to +2.99; very moist: +3.00 to +3.99; extremely moist: +4.00 and above) to assess overall conditions in South Texas.
Host and helminth collection
Bobwhites were donated by cooperators during the hunting seasons of 2014–2015 (25 October–22 February) and 2015–2016 (31 October–28 February). Cooperators were asked to place harvested bobwhites immediately in individual plastic bags labelled with location and date, and store on ice in coolers until they could be placed in a freezer at the end of each morning or afternoon hunt. Frozen carcasses were transported to and stored in freezers at the Buddy Temple Wildlife Pathology and Diagnostic Laboratory in Kingsville, Texas.
Each bobwhite was thawed overnight, massed using a calibrated scale, aged (juvenile or adult) and sexed using plumage characteristics and necropsied (see Shea (Reference Shea2016) for specific details on necropsy procedures). Nematodes were fixed in glacial acetic acid prior to storage in a solution containing 70% ethyl alcohol and 8% glycerine. Cestodes and acanthocephalans were fixed in acid–formalin–ethyl alcohol prior to storage in 70% ethyl alcohol. For identification and counting, nematode specimens were placed on ethyl alcohol–glycerine wet-mounted microscope slides and examined using a dissection scope (1–40×) and a compound light microscope (100× and 400×). When necessary for identification, cestodes and acanthocephalans were stained with Harris Haematoxylin containing an Eosin counterstain, mounted in Canada balsam on microscope slides and examined under magnification (compound light microscope, 100× and 400×). Cestodes were counted based on the number of scoleces recovered. If no scolex was found and there was uncertainty as to the occurrence of more than one cestode of the same species within the same host individual, intensity was assumed (conservatively) to be one. Voucher specimens were deposited at the Sam Houston State University Parasite Museum, Sam Houston State University, Huntsville, Texas (accession numbers SHSUP001587–SHSUP001598). Parasitological terms follow recommendations of Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997), where intensity was used for infrapopulation infection within an individual host and abundance was used to describe component population infections within the host sample (includes infected and uninfected hosts). Helminth species were defined as common (≥75% prevalence), intermediate (≥25 and <75% prevalence) or rare (<25% prevalence) following Landgrebe et al. (Reference Landgrebe, Vasquez, Bradley, Fedynich, Lerich and Kinsella2007).
Statistical analyses
Analyses were conducted using Statistical Analysis Software (SAS) 9.3 (SAS Institute Inc., Cary, North Carolina, USA) on the common helminth species to evaluate the effects of host intrinsic (age, sex, mass) and extrinsic (year ‘hunting season’) variables. Prevalence data for age, sex and year were analysed using a generalized linear model fitted with a binomial distribution and a logit link function. Abundance data were analysed using a generalized linear model and a log link function for a negative binomial response distribution where the independent variables were host age, host sex and year; the effects of body mass were tested by adding this variable as a continuous covariable (independent variable). An analysis of variance test was used to compare mean masses between non-infected and infected birds. Normality of residuals was tested with the Shapiro–Wilk test and the Welch's test was used when there were heterogeneous variances. Level of significance for statistical analyses was set at α < 0.05. Descriptive statistics are presented as a mean ± standard error.
Two similarity indices were used to evaluate differentiation diversity (β) of helminth component communities for the main effects of host age, sex and year. The Jaccard's coefficient of similarity index (JCi; Magurran, Reference Magurran2004) assessed the numerical similarity of shared species between component communities, and the percent similarity index (PSi; Krebs, Reference Krebs1999) was used to measure similarity of species’ abundances between component communities standardized as percentages. These indices were also used to compare overall helminth component communities between our study that occurred during a wetter period in South Texas to that of Olsen & Fedynich (Reference Olsen and Fedynich2016), which occurred during a drier period. Species abundances in our study were ranked from high to low to qualitatively assess numerical dominance.
Results
Three hundred and fifty-six bobwhite carcasses were donated by cooperators during the 2014–2015 (n = 124; 64 females, 60 males; 67 juveniles, 57 adults) and 2015–2016 (n = 232; 108 females, 124 males; 149 juveniles, 83 adults) hunting seasons. Ten helminth species were found and accounted for 14,127 individuals (table 1). Nematodes were the most common taxonomic group followed by cestodes and acanthocephalans (table 1). Helminths were found in the ceca, crop, eye surface, intra-orbital glands and ducts, heart pericardium, large intestine, neck muscle, proventriculus and small intestine (table 1).
Table 1. Descriptive statistics of helminths ranked by abundance (high to low) from 356 northern bobwhites (Colinus virginianus) collected during the 2014–2015 and 2015–2016 hunting seasons in South Texas.
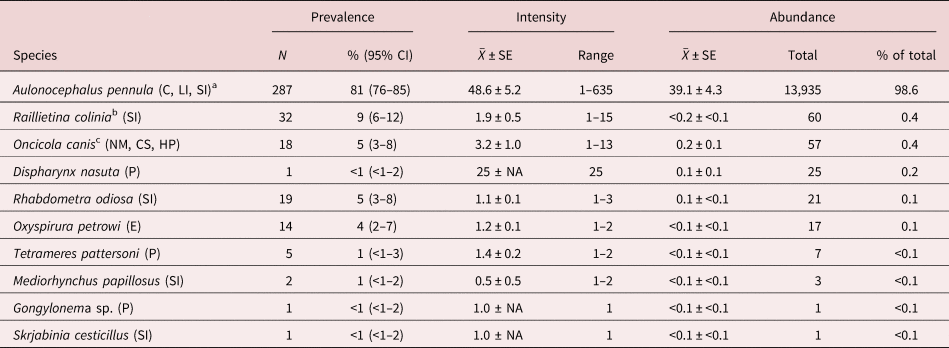
SE, standard error; CI, confidence interval.
a Helminth microhabitat: C, ceca; CS, crop serosa; E, eye surface, infraorbital glands and ducts; HP, heart pericardium; LI, large intestine; NM, neck muscle; P, proventriculus; SI, small intestine.
b Includes 20 individual bobwhites that were infected with 20 Raillietina individuals that could not be identified to species because of poor quality of specimens.
c Based on findings of Olsen (Reference Olsen2014).
Aulonocephalus pennula was the most common and abundant species, occurring in 287 of the 356 (81%) bobwhites and accounting for 13,935 (99%) of the total helminth individuals at the component community level (table 1). Each of the remaining species occurred rarely (≤9% prevalence) and contributed few individuals (≤0.4%; table 1). Dispharynx nasuta and Gongylonema sp. are reported for the first time from quail in South Texas. Species found that are known to be pathogenic in bobwhite included O. petrowi, Tetrameres pattersoni and D. nasuta, which occurred in 4%, 1% and <1% of the bobwhites, respectively (table 1).
At the infracommunity level, infections averaged 1.1 ± <0.1 species and ranged from 1–4 species (fig. 2). Intensity of infection of A. pennula ranged from 1 to 635 individuals, whereas intensity in the remaining species ranged from 1 to 25 individuals (table 1). Most (66–81%) individual bobwhites infected with A. pennula had 1–50 individuals, whereas few (<1%) A. pennula-infected bobwhites had many (>500; fig. 3).
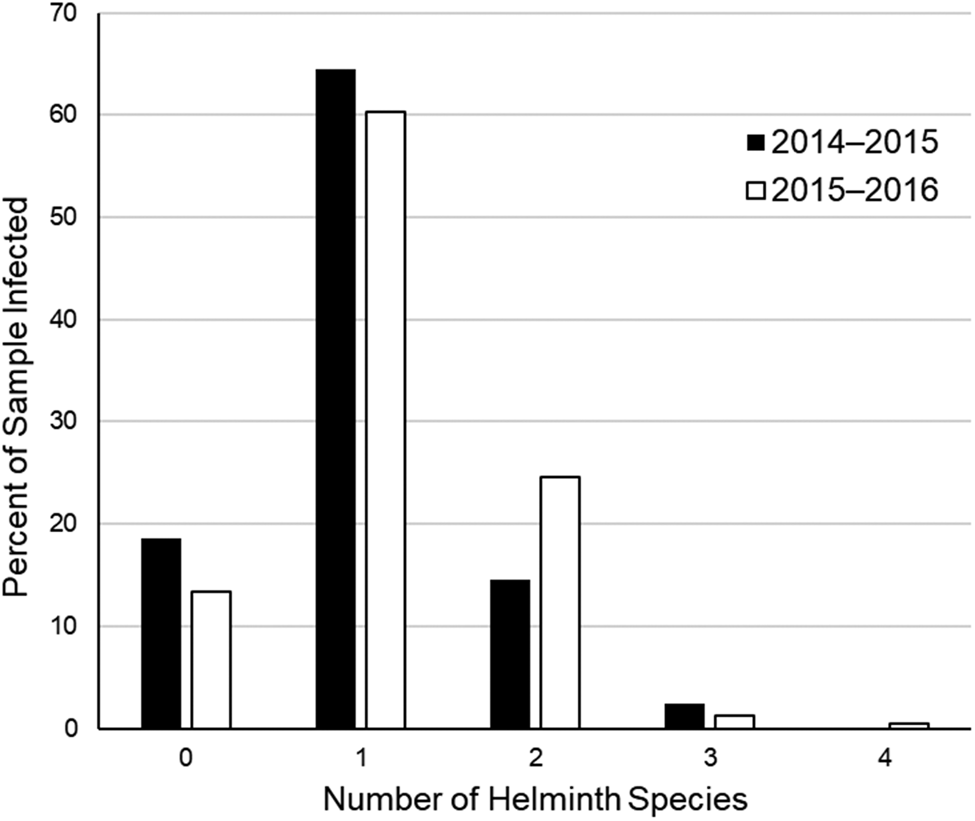
Fig. 2. Percent distribution of number of helminth species found in 124 northern bobwhites (Colinus virginianus) collected during the 2014–2015 hunting season and 232 northern bobwhites collected during the 2015–2016 hunting season in South Texas.
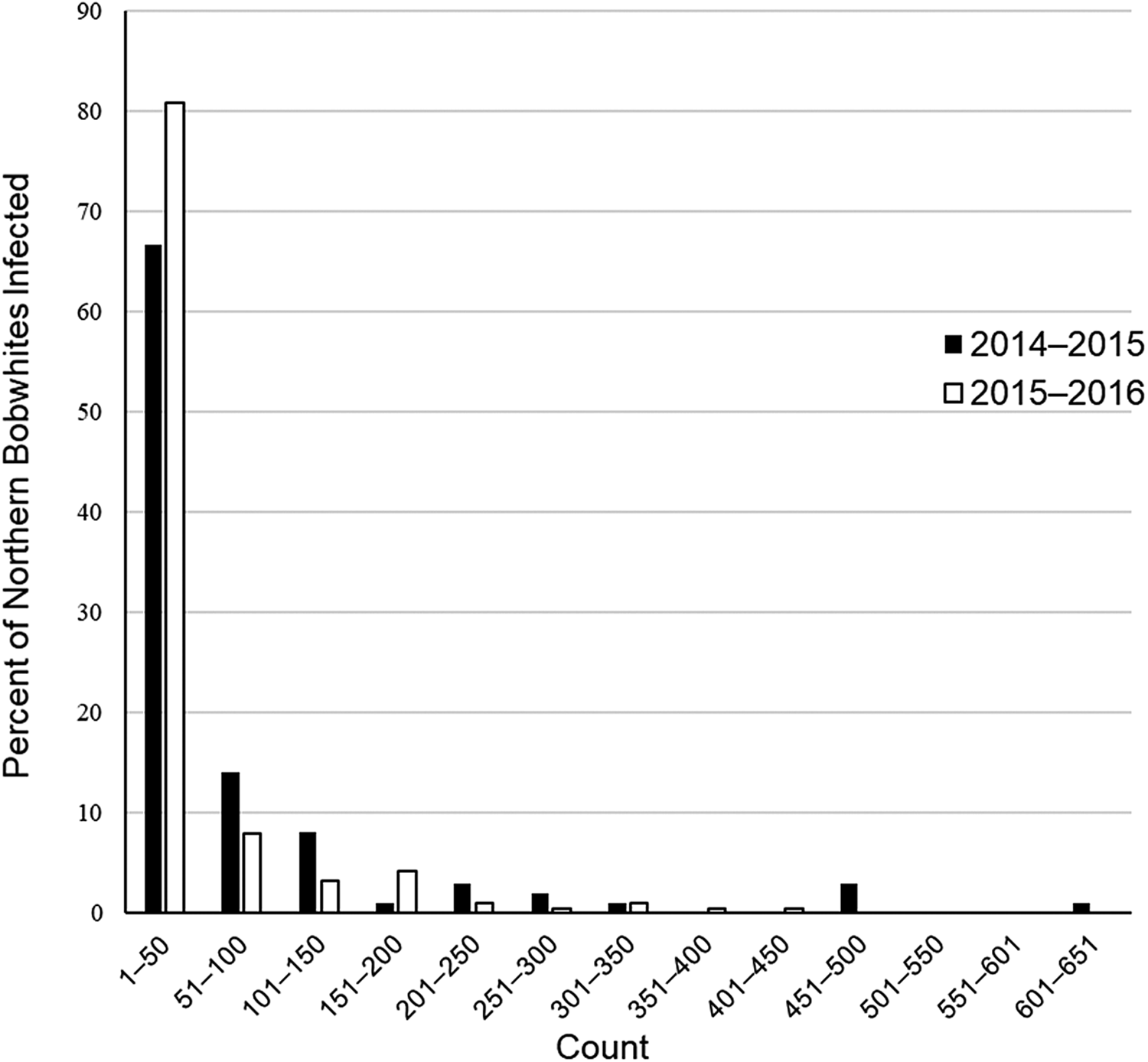
Fig. 3. Percent distribution of Aulonocephalus pennula infecting 99 northern bobwhites (Colinus virginianus) collected during the 2014–2015 hunting season and 188 bobwhites collected during the 2015–2016 hunting season in South Texas.
Influence on host age, sex, mass and year
Aulonocephalus pennula was the only common helminth species in which statistical analysis could be performed. Prevalence and mean abundance of A. pennula did not differ for host age, host sex or host body mass (table 2). Additionally, there was no difference between the mean body mass of infected and non-infected bobwhites (table 2). Prevalence of A. pennula did not differ by year; however, bobwhites collected during the 2014–2015 hunting season had a significantly higher mean abundance of A. pennula (53.7 ± 8.8) than those collected during the 2015–2016 hunting season (31.7 ± 3.8; table 2).
Table 2. Estimated prevalence and mean abundance of Aulonocephalus pennula with associated statistical metrics by host age, host sex and year of infection from 356 bobwhites (Colinus virginianus) collected during the 2014–2015 and 2015–2016 hunting seasons in South Texas.
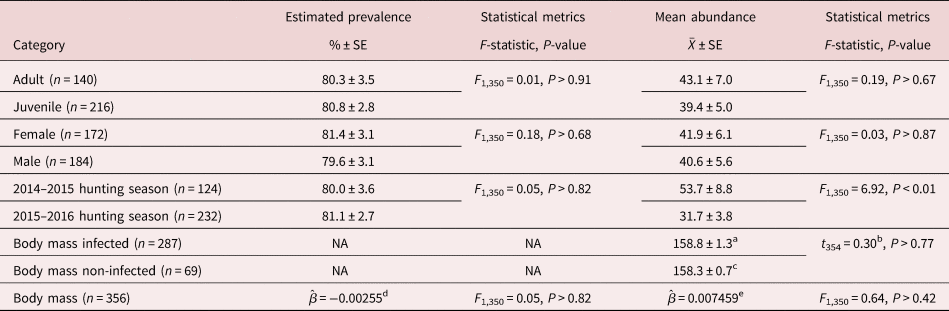
SE, standard error.
a Represents mean body mass in grams of infected individuals ± SE.
b T-statistic value comparing mean body mass of infected vs non-infected individuals.
c Represents mean body mass in grams of non-infected individuals ± SE.
d Effect of host body mass on estimated prevalence represented by the coefficient value in the generalized linear model.
e Effect of host body mass on estimated mean abundance represented by the coefficient value in the generalized linear model.
Noticeable differences in species composition were observed using JCi between cohorts of host age, sex and year (table 3). However, values for PSi of host age, sex and year were identical at 0.99 (table 3). Overall helminth component community comparisons to that of Olsen & Fedynich (Reference Olsen and Fedynich2016) found disparity in number of shared species (JCi = 0.5; six of the 13 species found in the two studies co-occurred), whereas both studies showed similarity in species’ distributions (PSi = 96.4; table 3).
Table 3. Jaccard's coefficient of similarity (JCi) and percent similarity (PSi) indices for helminth component community comparisons within each cohort of host age, sex and year of collection in 356 northern bobwhites (Colinus virginianus) collected during the 2014–2015 and 2015–2016 hunting seasons in South Texas, and overall helminth component community comparisons between the current study and Olsen & Fedynich (Reference Olsen and Fedynich2016).
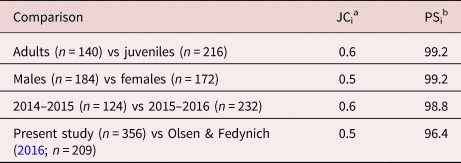
a Values for JCi range from 0 to 1, where 0 = completely dissimilar communities and 1 = completely similar communities.
b Values for PSi range from 0 to 100, where 0 = completely dissimilar communities and 100 = completely similar communities.
Severe drought conditions (average PDSI for South Texas: −3.00 to −3.99) occurred during April–September 2012 and 2013 during the study of Olsen & Fedynich (Reference Olsen and Fedynich2016). Conditions began to improve April–September 2014 (−1.99 to +1.99) and continued during April–September 2015 (+3.00 to +3.99).
Discussion
The helminth component community consisted of ten species. While all species were heteroxenous, only one species, A. pennula, demonstrated high prevalence, abundance and was numerically dominant (table 1). This likely precluded finding differences in A. pennula across host age, sex and mass.
The effect of numerical dominance also was observed in component community comparisons of PSi and with findings of Olsen & Fedynich (Reference Olsen and Fedynich2016; table 3), which reduced the usefulness of PSi in measuring diversity. A similar trend of A. pennula numerical dominance within the component community and high prevalence within bobwhite populations is apparent in the Rolling Plains as well, though abundance has been found to be about 2–3 times higher in recent community-wide surveys in the Rolling Plains (84.9 ± 9.1 to 128.6 ± 14.9; Villarreal et al., Reference Villarreal, Bruno, Fedynich, Brennan and Rollins2016; Bruno et al., Reference Bruno, Fedynich, Rollins and Wester2018, Reference Bruno, Rollins, Wester and Fedynich2019) compared with the present study (39.1 ± 4.3) and that of Olsen & Fedynich (Reference Olsen and Fedynich2016, 64.1 ± 6.0) from South Texas. While some of these studies span the same years and are consistent in sampling scheme and season of collection, others vary in their study design and sampling years, making interpretation regarding varying abundance difficult.
It is uncertain why A. pennula is so successful in Texas quail populations. Aulonocephalus pennula is an imbiber (Inglis, Reference Inglis1958) and does not seem to attach to host caecal tissues (Olsen et al., Reference Olsen, Brennan and Fedynich2016; personal observations by authors), which could limit host immune response. Second, the intermediate host is presently thought to be an insect (Henry et al., Reference Henry, Brym, Kalyanasundaram and Kendall2018; Peterson & Fedynich, Reference Peterson, Fedynich and Brennanin press) that is likely abundant, comprises a major portion of the bobwhite's insect diet (insects represent 8–23% of the total diet; Larson et al., Reference Larson, Fulbright, Brennan, Hernández and Bryant2010) and is a highly suitable host for the larval stages of this helminth. In addition, there appeared to be a precipitation effect when comparing the present dataset to that of Olsen & Fedynich (Reference Olsen and Fedynich2016), whose study occurred during severe drought conditions. Intensity of A. pennula decreased between the collection periods spanning the hunting seasons of 2012–2014 (82.2 ± 7.1) and 2014–2016 (48.6 ± 5.2), whereas prevalence remained relatively consistent (81% and 78%, respectively). Possibly, the proportion of infected intermediate hosts decreased within their respective intermediate host populations as those populations increased in response to favourable environmental conditions. Thus, as the ratio of A. pennula-infected intermediate hosts to uninfected intermediate hosts decreased, intensity also decreased.
The pattern of a single common (>75% prevalence) species with the remaining species predominately being rare (<25% prevalence), or with a single species being intermediate (≥ 25 and <75% prevalence), has been observed in large helminth community studies (host sample sizes >100) of scaled quail (Callipepla squamata) in western Texas (Fedynich et al., Reference Fedynich, Bedford, Rollins and Wester2020) and bobwhites in South Texas (Olsen & Fedynich, Reference Olsen and Fedynich2016) and the Rolling Plains of Texas (Villarreal et al., Reference Villarreal, Bruno, Fedynich, Brennan and Rollins2016; Bruno et al., Reference Bruno, Fedynich, Rollins and Wester2018, Reference Bruno, Rollins, Wester and Fedynich2019). In addition, our findings and that of Olsen & Fedynich (Reference Olsen and Fedynich2016) contrast with findings from the southeastern US in which there was a more distributed pattern of occurrence with reports of 11 helminth species (three intermediate, eight rare) in a sample of 71 bobwhites from Georgia, Florida and South Carolina (Kellogg & Prestwood, Reference Kellogg and Prestwood1968), 15 species (four common, three intermediate, eight rare) in a sample of 185, 12 species (four common, one intermediate, seven rare) in a sample of 153, and 15 species (three common, two intermediate, ten rare) in a sample of 700 from Leon County, Florida (Davidson et al., Reference Davidson, Kellogg and Doster1980; Moore et al., Reference Moore, Freehling and Simberloff1986; Davidson et al., Reference Davidson, Kellogg, Doster and Moore1991, respectively).
Besides observing a difference in the frequency distribution of helminth species compared with the south-eastern edge of the bobwhite range, a notable difference in helminth lifecycle transmission strategy was identified. At least eight monoxenous species are known to occur in the south-eastern US (Davidson et al., Reference Davidson, Kellogg and Doster1982; Forrester & Spalding, Reference Forrester, Spalding, Forrester and Spalding2003), which were absent in bobwhites from South Texas or limited to one to two species (Trichostrongylus tenuis = Trichostrongylus cramae; Demarais et al., Reference Demarais, Everett and Pons1987) occurring in wetter regions (e.g. Gulf Coast Prairies and Marshes ecoregion) that extend into coastal areas of South Texas and coastal areas in the eastern portion of South Texas (T. cramae and Strongyloides avium; Purvis et al., Reference Purvis, Peterson, Dronen, Lichtenfels and Silvy1998). Consequently, there seems to be a link between wetter regimes and the presence and persistence of monoxenous species. Although the southeastern US climate is humid subtropical, South Texas experiences local climates of humid subtropical and semi-arid. However, unpredictable rainfall in South Texas, ranging from droughts to severe floods, results in the dramatic boom-and-bust cycle of bobwhites (Hernández et al., Reference Hernández, Guthery and Kuvlesky2002), which occurs with much less magnitude in the southeastern US. These factors may negatively impact transmission potential of monoxenous species and favour heteroxenous species that can maintain infective stages within intermediate hosts during extreme environmental periods (Olsen & Fedynich, Reference Olsen and Fedynich2016). Consequently, South Texas may be incapable of supporting a helminth community in bobwhites similar in structure and pattern relative to the southeastern US.
Several rare species (Raillietina colinia, Rhabdometra odiosa, D. nasuta and Gongylonema sp.) were found in our study, which were absent in the study of Olsen & Fedynich (Reference Olsen and Fedynich2016), whereas two rare species (Physaloptera sp. and Fuhrmannetta sp.) were reported in Olsen & Fedynich (Reference Olsen and Fedynich2016) and not found in the present study (reflected in differences of JCi values of component communities; table 3). In addition, T. pattersoni and O. petrowi occurred infrequently (<4% prevalence) and at low intensities of one or two individuals per infected bird, whereas Olsen & Fedynich (Reference Olsen and Fedynich2016) found a higher prevalence (10%) and higher (2.8 ± 0.7) mean intensity of T. pattersoni, and a higher prevalence (9%) and higher mean intensity (4.9 ± 1.7) of O. petrowi. It is unclear whether these rare species are responding to factors related to precipitation. It may be more likely that the variation observed is related to sampling effort (Poulin, Reference Poulin1998), varying levels of detection probability among years due to fluctuating prevalence of rarely occurring species, and the probability of an infected host with a rare species being sampled.
Clearly, long-term studies are needed to understand the ecology and detection dynamics of rare species within South Texas. Further research is also warranted to determine how intermediate host population cycles react in environments with stochastic precipitation regimes and to determine prevalence and intensity of infections of intermediate hosts as bobwhites experience boom-and-bust cycles.
Pathogenic species
Tetrameres pattersoni, O. petrowi and D. nasuta were found, which are known to cause morbidity (Davidson et al., Reference Davidson, Kellogg, Doster and Moore1991; Bruno et al., Reference Bruno, Fedynich, Smith-Herron and Rollins2015; Dunham et al., Reference Dunham, Bruno, Almas, Rollins, Fedynich, Presley and Kendall2016; Olsen & Fedynich, Reference Olsen and Fedynich2016; Brym et al., Reference Brym, Henry and Kendall2018; Bruno et al., Reference Bruno, Rollins, Wester and Fedynich2019) and mortality (Kellogg & Prestwood, Reference Kellogg and Prestwood1968) in bobwhites. In South Texas, only T. pattersoni and O. petrowi were found (Olsen & Fedynich, Reference Olsen and Fedynich2016) prior to the present study in which D. nasuta is reported for the first time in South Texas. Our findings in South Texas contrast with recent surveys of helminth communities in the Rolling Plains, which report higher frequency (ranging 14–26%) and similar mean intensity (2.9 ± 0.3 to 3.8 ± 0.1) of T. pattersoni and higher frequency (ranging 40–67%) and higher mean intensity (5.6 ± 0.7 to 14.2 ± 0.2) of O. petrowi (Villarreal et al., Reference Villarreal, Bruno, Fedynich, Brennan and Rollins2016; Bruno et al., Reference Bruno, Fedynich, Rollins and Wester2018, Reference Bruno, Rollins, Wester and Fedynich2019). In addition, studies that focused on O. petrowi infections have reported intermediate to high (up to 95%) prevalence in the Rolling Plains ecoregion (Dunham et al., Reference Dunham, Bruno, Almas, Rollins, Fedynich, Presley and Kendall2016, Reference Dunham, Peper, Downing, Brake, Rollins and Kendall2017; Dunham & Kendall, Reference Dunham and Kendall2017). However, D. nasuta appears to be rare (<1%) in bobwhites within the Rolling Plains (Villarreal et al., Reference Villarreal, Bruno, Fedynich, Brennan and Rollins2016; Bruno et al., Reference Bruno, Fedynich, Rollins and Wester2018, Reference Bruno, Rollins, Wester and Fedynich2019) and South Texas (table 1). As noted previously, variation in study designs makes it difficult to draw conclusions from direct comparisons, though trends do seem apparent.
The dichotomy between geographic regions in Texas suggests that suitable intermediate hosts for T. pattersoni and O. petrowi in South Texas are not as prevalent as found in the Rolling Plains or that these helminths have difficulty in persistence or transmission within the South Texas landscape. Unfortunately, little is known about the ecology of pathogenic species in South Texas, which is needed to understand geographic variation within Texas. Regardless, the occurrence of pathogenic helminth species in Texas suggests that individual bobwhites have the potential to be negatively impacted. However, these species do not seem to occur with sufficient frequency or intensity to be a population regulator of bobwhites in South Texas.
Acknowledgements
We appreciate the help of numerous landowners, ranch managers and hunters who donated harvested bobwhites for parasite research. We are grateful to Dr Mike Kinsella for his expertise with helminth identifications and Dr Autumn Smith-Herron for providing help with staining protocols. We thank Megan Hess for her help making fig. 1 of this manuscript. We also thank the reviewers for their helpful suggestions and feedback. This is manuscript number 20-133 of the Caesar Kleberg Wildlife Research Institute.
Financial support
The René R. Barrientos Fund for Graduate Student Tuition provided support to S.A. Shea. This research was funded by the South Texas Chapter of Quail Coalition and the Caesar Kleberg Wildlife Research Institute.
Conflicts of interest
None.
Ethical standards
Bobwhite collection was approved by Texas Parks and Wildlife (SPR-0498-949) and research was conducted in accordance with Texas A&M University-Kingsville Institutional Animal Care and Use Committee (2012-09-29A) and Texas A&M University-Kingsville Institutional Biosafety Committee (2012-11-28-IBC-C-A2).








