Tue thermal conductivity of pure polycrystalline ice at 0°C is approximately four times that of water at that temperature. The international critical tables contain two values for ice at 0°C which differ by 5%. Reference Sehofield, Hall and WashburnSchofield and Hall (1927) selected a value of 2.20 W m-1 deg-1, whereas Reference Dusen and WashburnVan Dusen (1929) gave a value of 2.09 W -1mdeg-1. Subsequent measurements as listed by Reference PowellPowell (1958) have confirmed that the I.C.T. values are approximately correct and have suggested that crystal anisotropy could possibly account for the observed small differences. The position is much less satisfactory at lower temperatures where large differences exist between the results of Reference LeesLees (1905) and Reference Jakob and ErkJakob and Erk (1929). The latter’s results appear to be more reliable, since Reference Dillard and TimmerhausDillard and Timmerhaus (1966) have reproduced these values experimentally and Reference RatcliffeRatcliffe’s (1962) values also agree to within about 12% at -120°C with those of Jakob and Erk. All these values are for polycrystalline ice. Moreover, the thermal conductivity of many crystalline materials has been found to be proportional to the reciprocal of the absolute temperature within a certain temperature range (Reference EukenEuken, 1911). Such a relation down to 100 K is more nearly satisfied by the data of Jakob and Erk than those of Lees, and also fits the data of Dillard and Timmerhaus and of Ratcliffe. Recently, Reference FletcherFletcher (1970) has described a “hump" effect in the temperature relationship at low temperatures, which has also been accurately measured by Reference Klinger and NeumaÏerKlinger and Neumaier (1969).
In snow, numerous measurements of the thermal conductivity exist close to the melting point. Summaries of these (Reference MantisMantis, 1951; Reference MellorMellor, 1964) show considerable divergence of the results owing partly to the difficulties in measuring low snow densities correctly and in neglecting structural differences in snow samples, and convective and radiative heat transfer processes in the snow. Theoretical attempts to formulate the thermal conductivity of snow as a function of density include a recent paper by Reference SchwerdtfegerSchwerdtfeger (1963). No data on the temperature coefficients are available.
The re-analysis of earlier measurements (Reference SehyttSchytt, 1958) of subsurface temperatures at Maudheim, Antarctica has provided a new value of the thermal conductivity of ice of density 0.57 Mg m-1 (Reference Dalrymple, Dalrymple, Lettau, Wollaston and RubinDalrymple and others, 1966). Similarly, a recent analysis of annual sub-surface temperatures at “Plateau" station on the central Antarctic plateau has given a new value of the thermal conductivity of snow of density 0.42 Mg m-3 (Reference Weller and SchwerddcgerWeller and Schwerdtfeger, 1968). These two new values were determined from Fourier-type analyses at mean annual snow temperatures of - 17°C and 60°C respectively, at depths where non-conductive processes of heat transfer were shown to be negligible. By comparing these results, listed in the table below, with mean weighted values of a series of measurements close to the freezing point, taken from Reference MellorMellor (1964), the temperature coefficients of the thermal conductivities and diffusivities could be deduced. They are assumed to be linear over the temperature range considered, as shown approximately by the laboratory results of Reference Jakob and ErkJakob and Erk (1929), Reference Dillard and TimmerhausDillard and Timmerhaus (1966), and Reference RatcliffeRatcliffe (1962). Changes of specific heat of the ice with temperature were taken from Reference DorseyDorsey (1940).
Table I. Tiif.rmal propiïkîiks of tcf.
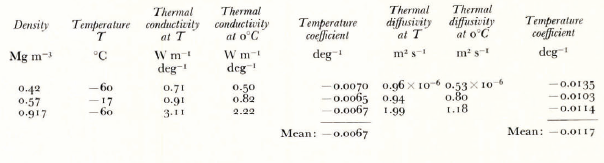
There is reasonable agreement between the temperature coefficients at different densities, and this further confirms the values obtained by Jakob and Erk.
The temperature dependence of the thermal properties can be seen to be anything but negligible, even for small temperature changes near the freezing point. This is usually not considered in heat-flux computations in ice and snow and may lead to considerable errors.



