Introduction and Literature Review
The growth forms of ice in supercooled water and aqueous solutions have been studied by various investigators. The purpose of this section is to summarize briefly the results of investigations reported in the literature.
Reference Kumai and ItagakiKumai and Itagaki (1953) examined the growth forms of ice crystals in the surface of supercooled water by a motion-picture technique. Their results showed that at supercoolings smaller than 1°C. crystals grew as circular discs which often developed notches at the edges. The crystallographic c-axes of these discs were found to be perpendicular to the plane of the discs. At supercoolings larger than 1°C. six-armed dendrites developed from the discs which grew so rapidly that the disc stage often could not be recognized. If a disc was inclined at an angle to the surface, growth proceeded in the liquid surface from the edge of the disc in the form of a long thin needle. Reference HallettHallett (1960) examined the mode of growth of ice crystals in a water surface in greater detail. He showed that ice crystals grown on the surface of supercooled water had a composite structure which he called “surface needle”. Each such needle consisted of dendrites growing into the liquid bulk and of ribs growing in the liquid surface. Each needle was a single crystal with its optic axis perpendicular to the plane of the dendrite.
Reference KnightKnight (1966) made a series of laboratory investigations in an attempt to explain the preferred ice-crystal orientation found in lake ice which even in different parts of the same lake varied from c-axis vertical to c-axis horizontal to random. With the air temperature set between −13 and −16°C., Knight grew sheets of ice about 3 cm. thickness in the surface of pure water and he investigated them between crossed polaroids. He found that the c-axes of the individual ice crystallites of the polycrystalline sheet of ice were mostly horizontally orientated. By plotting the length of the inter-grain boundary against the angular difference in c-axis orientation between adjacent crystallites, he obtained maxima at 15, 35 and 90 angular degrees. Knight concluded that the final preferred crystal orientation was affected by the initial orientation, by selected wedging cut of grains of certain orientations and often by grain-boundary migration. Reference KnightKnight (1962) studied the dendritic growth of ice on glass, metal and plastic surfaces. He found that the ice dendrites which grew from supercooled water on these surfaces changed their orientation in response to asymmetrical temperature gradients. The growth was stable only when the c-axes of the growing ice crystals were perpendicular to the surface. Increased initial supercooling caused the curved growth to be more pronounced while dissolved sodium chloride inhibited it.
Reference CampCamp (1965) studied the growth forms of ice on glass, lucite and aluminium by a photographic method. He showed that on these surfaces they were different from each other. Most of the growth forms were curved and could be divided into three classes: those having their c-axes approximately perpendicular to the surface, those having their c-axes making a small angle with the surface and those which changed orientation as they grew. Camp concluded that a knowledge of the thermal properties of the substrate alone did not suffice to explain his observations.
Reference HallettHallett (1964) used a motion-picture technique to study the growth of ice crystals in supercooled water which was contained in Pyrex vessels of volumes between 1.0 and 0.1 cm.3 submerged in a cold bath. Freezing was initiated by a pre-grown single ice crystal. Viewing the growing crystals perpendicular to the basal plane of the seed crystals, he found that at supercoolings between 0 and 5°C. growth took place in the form of a thin dendritic sheet of ice along the direction of the basal plane of the seed crystal. At lower temperatures other orientations were nucleated as the seed crystals entered the supercooled water drop and three-dimensional ice structures were formed. Drops which had diameters between 1,500 and 500 μ and which were supercooled between 0 and 5°C. froze on a single ice crystal with the same crystal orientation as the substrate. At supercoolings between 5 and 15°C. the drops froze polycrystalline with the c-axes of most crystallites either parallel or at 90° to the c-axis of the ice substrate. At supercoolings between 15 and 25°C. the orientations of the crystallites were no longer simply related to the ice substrate. At supercoolings of 6°C. about two to four crystals were formed in a frozen drop, at supercoolings of 15°C. about five to 15 crystals, and at supercoolings of 20°C. about 30 to 70 single crystals were formed in a frozen drop.
Reference ListList (1958, Reference List1960) and Reference AufdermaurAufdermaur and others (1963) found that the ice of most natural hailstones is polycrystalline despite the fact that most hailstones originate on single ice crystals from which they grow by accretion of supercooled water drops. They found that ice crystallites in a hailstone were large when the growth temperature of the hailstone was close to 0°C. and they were small when the growth temperature of the hailstone was close to the ambient air temperature, which is considerably below 0°C. in a cloud in which hail is developed. In most cases the individual crystallites were found to be orientated eitherwith their c-axes in the growth direction or at random. In a few cases the crystallites were orientated perpendicular to the growth direction of the hailstone.
Reference LindenmeyerLindenmeyer (unpublished) and Reference Macklin and RyanMacklin and Ryan (1965) studied the free growth of ice in bulk supercooled water at supercoolings between 2.0 and 6.5°C. Lindenmeyer (unpublished) measured the growth rate and the growth forms of ice in supercooled water and supercooled aqueous solutions of sugar, acetic acid and potassium chloride. The water or a particular solution was contained in a glass vessel with a volume of 2 l which was submerged in a cold bath. Freezing in the stirred supercooled water or solution was initiated by means of small pieces of dry ice. Reference Macklin and RyanMacklin and Ryan (1965) determined the growth forms of ice in supercooled water and sugar solutions which were contained in cubic Plexiglass vessels of 4.2 cm. internal dimensions submerged in a cold bath. Freezing was initiated by pre-grown single ice crystals. Lindenmeyer, and Macklin and Ryan, observed that ice structures formed at supercoolings larger than 3°C. were not co-planar with the basal planes of the seed crystal but were split into two and occasionally more segments. Their photographs show that the split was symmetric with respect to the a- and the c-axes of the seed crystals. At supercoolings larger than 5.5°C. they observed secondary splitting on the major growth segments. The angle of split varied from 11 angular degrees at −3°C. to 30 angular degrees at −6°C. Lindenmeyer (unpublished) showed that the angle of split in a 0.1 molar sugar solution was larger than in a 0.1 molar potassium chloride solution. The angle of split in all three solutions was larger than that in pure water.
In a later section the present results of experiments on the growth modes of ice crystals in supercooled water and aqueous solutions are discussed and they are compared with the results quoted in the literature.
Experimental Procedure
In conjunction with an investigation of the growth rates of ice crystals in supercooled water and various aqueous solutions (which will be reported elsewhere) the growth forms of ice crystals were studied at supercoolings ranging from 0.5 to 20°C. The water used was purified by distillation and de-ionization according to the method described by Reference Pruppacher and NeiburgerPruppacher and Neiburger (1963). The aqueous solutions were prepared from this water by dissolving reagent-grade salts in it and filtering the solutions through Millipore filters of 0.1 μ pore size. The solutions, which had concentrations of 10−5, 10−4, 10−3, 10−2, 1×10−1, 2×10−1, 4×10−1, 6×10−1 and 8×10−1 moles l.−1, were prepared from the following substances: HF, NaF, KF, CsF, NH4F, LiCI, NaCI, KCl, CsCl, NH4Cl, LiBr, NaBr, KBr, CsBr and NH4Br.
In the first set-up the water or aqueous solutions were filled into polyethylene tubes of 1.2 mm. diameter, suspended horizontally and submerged in a continuously stirred cold bath of low-viscous silicone oil. The test section of the tube was 60 cm. long. After temperature equilibrium with the surrounding bath, freezing of the water or solution was initiated by touching one tube end sticking out of the bath with a piece of dry ice at a point about 20 cm. away from a test section. The growth modes of ice in the water and solutions were determined by a motion-picture technique. A Bolex motion-picture camera operated at 64 frames sec.−1 and Kodachrome II film were used in this technique. The growing ice crystals were filmed between crossed polaroids.
In a second set-up, water or solution drops of volumes 0.5 to 2.0 cm.3 were formed with a syringe and placed at the interface of Fluorochemical-75 and Squibb paraffin oil. Alternatively, the drops were placed at the interface of carbon tetrachloride and paraffin oil or placed on a hydrophobic surface in air.Footnote * The different environments of the drop did not alter the results in any way. After the drops had come into complete temperature equilibrium with the environment, crystallization was initiated by a single ice crystal which had the same temperature as the drop. The seed crystal had the form of a thin dendrite, was several millimeters in diameter and was grown by a special technique in slightly supercooled water. Each crystal was held by a specially constructed clip mounted on a micrometer device which was movable along three perpendicular directions. In this way one dendrite tip of the seed crystal could be brought exactly into the desired position for nucleating the supercooled drop. Each drop was nucleated with a new seed crystal. The seed crystal was mounted with its crystallographic c-axis either perpendicular or parallel to the plane of the motion-picture film. Best results were obtained with the latter orientation. The growth mode of ice in these supercooled water and solution drops was recorded by means of a Bolex motion-picture camera operated at 64 frames sec.−1 and Kodak Plus-X reversal film.
Results
By investigating the growth of ice crystals in water and aqueous solutions with the first set-up the following observations were made. At supercoolings smaller than 1°C. one ice dendrite and at supercoolings of 1 to 4°C. two ice dendrites were formed which grew along their crystallographic a-axes parallel to the tube axis. At supercoolings larger than 4°C. several ice dendrites formed whose direction of growth was inclined to the tube axis such that the dendrites hit the tube wall and afterward proceeded growing in a new direction. As a result it appeared that the ice crystals grew in a zigzag fashion which became more pronounced when the supercooling was increased or salts were dissolved in the water. With increased supercooling and addition of salts even in small concentrations the number of ice dendrites formed in water or solution increased. No specific action of different dissolved salts on the growth modes could be detected with the exception that in the solution of a few salts the ice dendrites followed, instead of a zigzag path, the path of a tightly wound screw. Such screw motion was observed in 0.1 molar potassium fluoride and sodium fluoride solutions at supercoolings between 6 and 8°C. Occasionally, screw motion was also observed in sodium chloride and potassium chloride solutions of a concentration of 0.1 mole 1.−1 or larger.
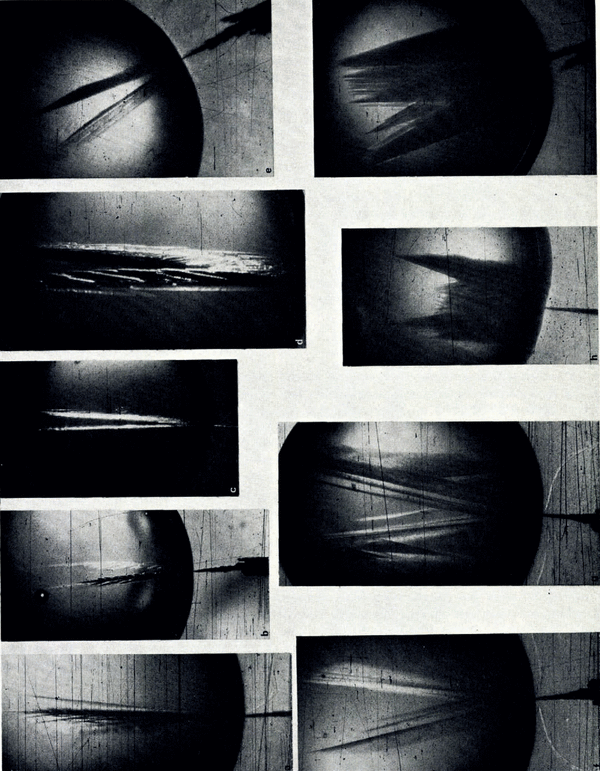
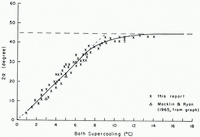
In an attempt to investigate the reason for the growth modes in capillary tubes, the growth modes of ice under free-growth conditions were carefully studied by means of the second experimental set-up. A selected number of photographs made from chosen frames of the motion-picture films are reproduced here to illustrate the arguments. Figure 1a–l is a series of photographs which show how the growth form of ice in pure water varied with increasing supercooling. It is seen from these photographs that the basic growth form of ice was dendritic at all tested supercoolings. Several modes of this dendritic growth, however, were observed which varied systematically with supercooling. At supercoolings smaller than about 1°C. the ice seed crystal continued its growth into the drop as a thin dendrite whose secondary branches grew slowly out to form a sheet of ice which was co-planar with the basal planes of the seed crystal. At supercoolings between about 1°C. and about 3.5°C. the ice seed crystal split into two thin dendritic segments whose secondary branches grew out in such a way that two sheets of ice were formed. These segments enclosed an angle which increased with increasing supercooling. The variation of the angle of split with supercooling is shown in Figure 2, in which it is seen that between a supercooling of 1 and 8°C. the angle of split varied almost linearly with supercooling. At supercoolings larger than 8°C. the angle of split increased less and at a supercooling of about 12°C. it reached a limiting value of about 45 angular degrees.
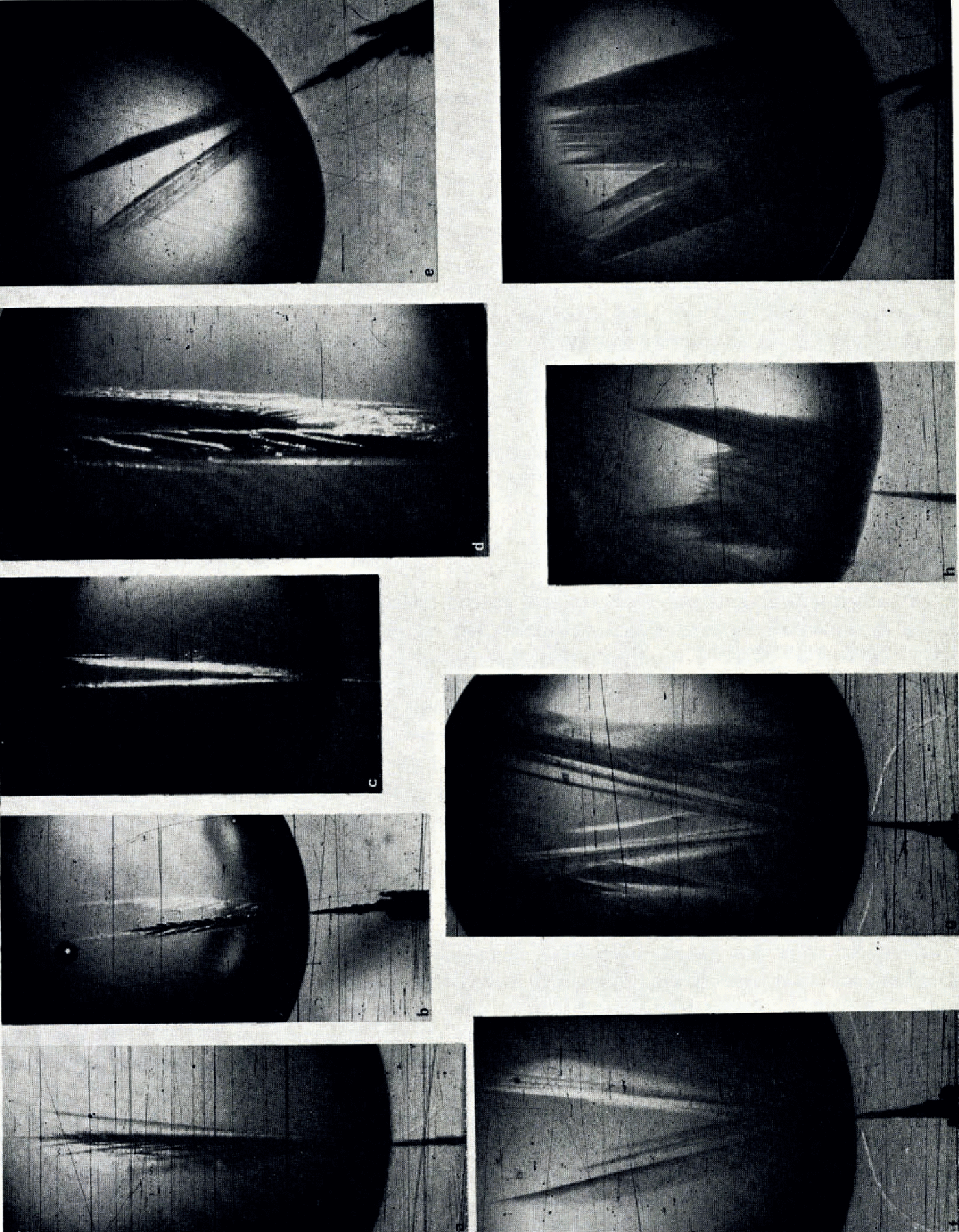
Fig. 1. Variation of ice-crystal habit with supercooling and salt content in drops 2 cm. in diameter (from 16 mm. motion picture film). The crystallographic c-axis of the seed crystal lies in the photographic plane. a. Pure water (supercooling 1°C.); b. Pure water (supercooling 2.6°C.); c. As b but after initial growth stopped; d. Detail of c; e. Pure water (supercooling 3.2°C.); f. Pure water (supercooling 3.9°C.); g. Pure water (supercooling 4.3°C.); h. Pure water (supercooling 5.5°C.); i. Pure water (supercooling 6.1°C.); j. Pure water (supercooling 9.0°C.); k. Pure water (supercooling 11.7°C.); l. Pure water (supercooling 9.1°C.); m. LiCI solution (0.1 molar; supercooling 2.6°C.); n. CsCI solution (0.1 molar; supercooling 3.0°C.):0. CsF solution (0.1 molar; supercooling 3.6°C.); p. CsCI solution (0.1 molar; supercooling 5.1°C.); q. KCl solution (0.8 molar; supercooling 8.0°C.)
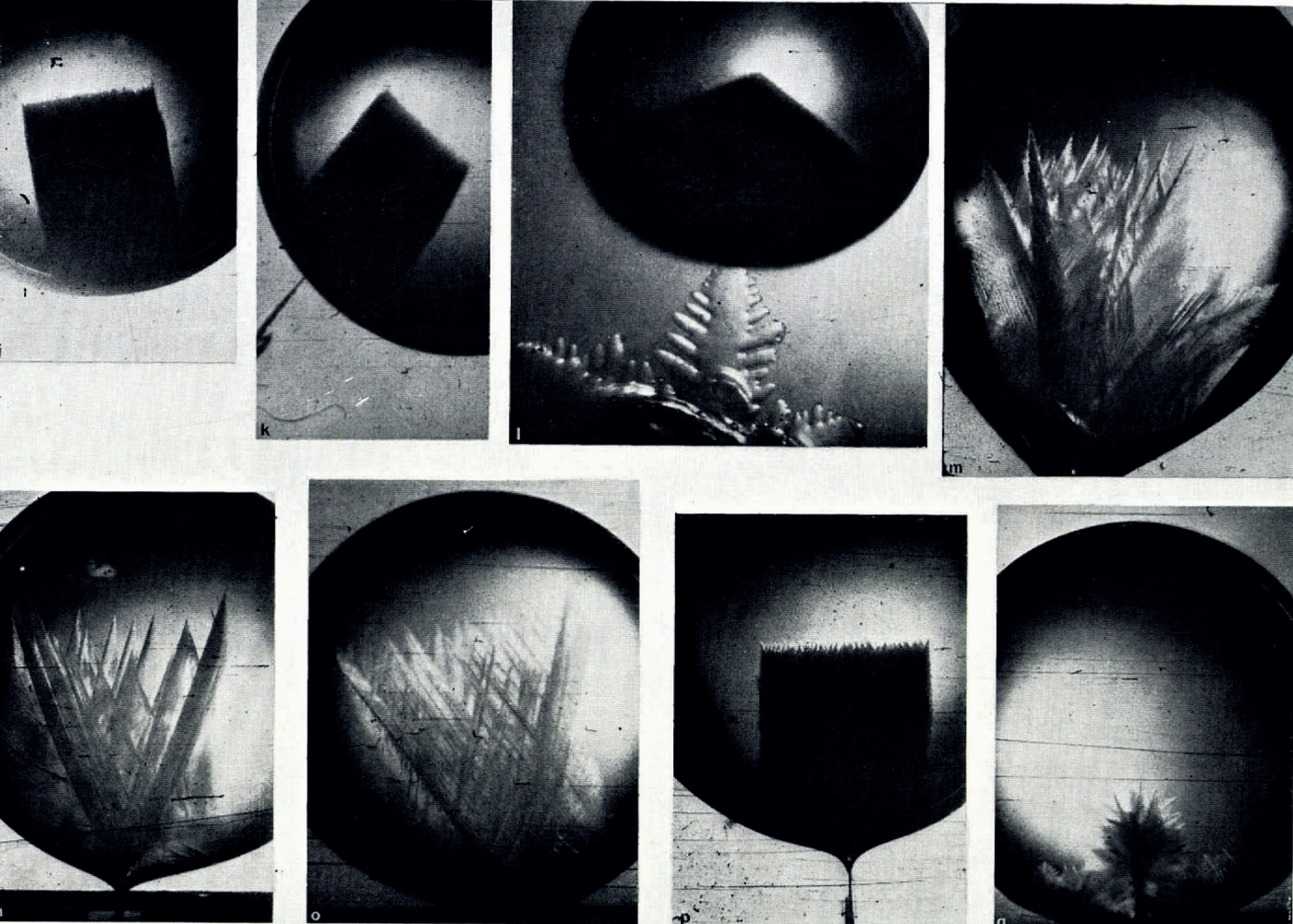
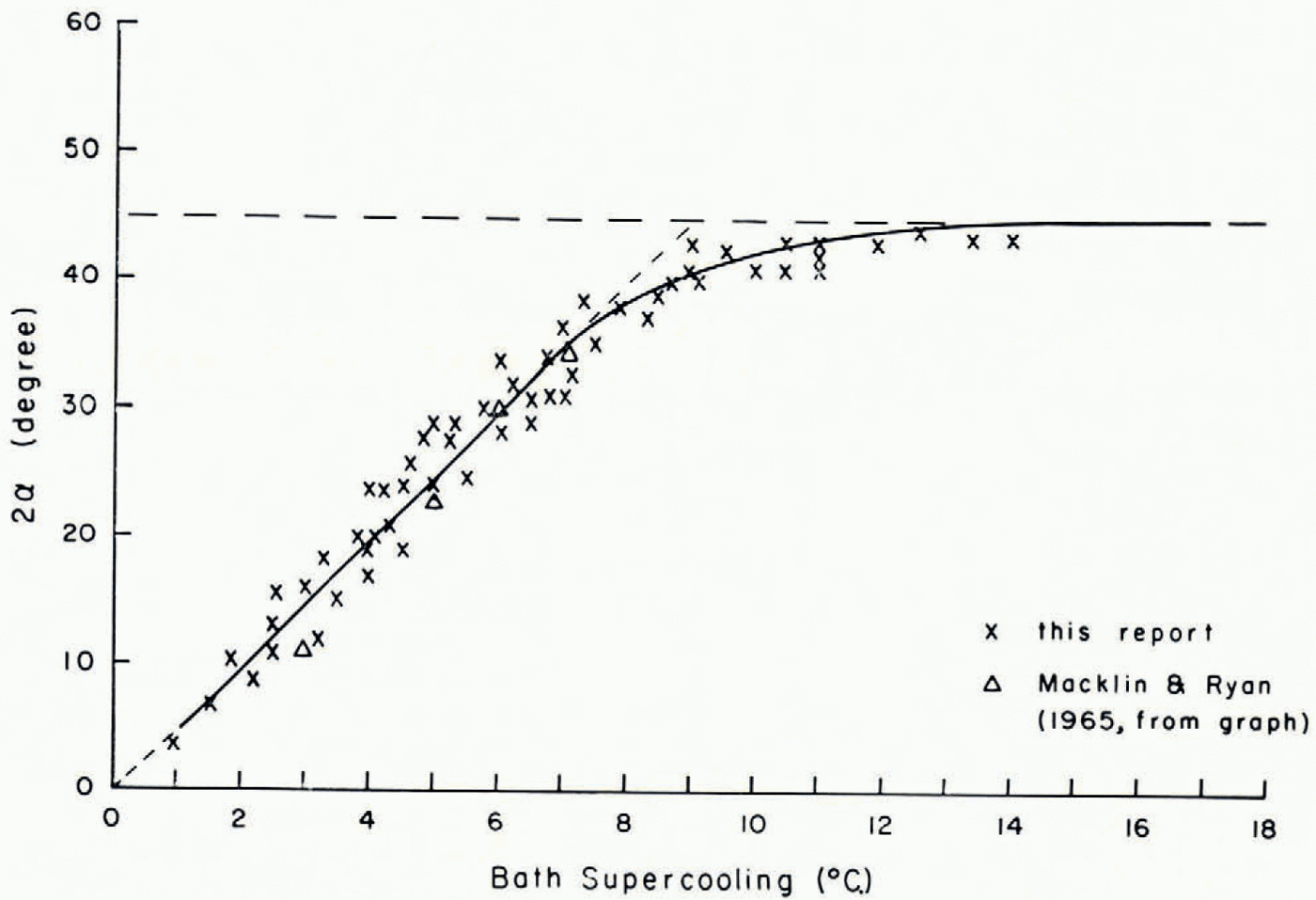
Fig. 2. Variation with supercooling of the angle 2α between the two principal growth directions of ice in pure water (α =angle between principal growth direction and basal plane of seed crystal)
At supercoolings between 3.5 and 5.0°C. the seed crystal still split into two primary dendritic segments. Each of these, however, underwent secondary and higher-order splitting to form a three-dimensional ice network. At supercoolings between about 5 and 9°C. the seed crystal itself underwent multiple splitting into dendritic segments, the number of which increased with increasing supercooling. At supercoolings larger than about 9°C., primary and higher-order segments with the side branches combined to form a three-dimensional ice network of dense mesh and rectangular growth front. When growth was initiated by a single ice crystal, whose c-axis was perpendicular to the photographic plane, the growth front was hexagonal as shown in Figure 1l.
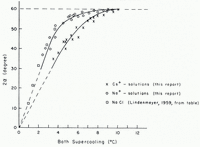
The variation of the growth modes of ice crystals in aqueous solutions with supercooling below the equilibrium freezing temperature of the solutions is illustrated by Figure 1m–q. The growth modes of ice crystals in solutions whose salt concentration was smaller than 10−3 mole l.−1 were the same as those in pure water. In solutions with salt concentrations larger than 10−3 mole l.−1 the growth modes were significantly altered. Instead of the five growth regimes observed in pure water only two growth regimes were found. In the first growth regime, at supercoolings between about 1 and 4°C., the seed crystal split into two major dendritic growth segments each of which underwent multiple splitting. A short time interval after the two primary segments had developed secondary or higher-order splitting took place on the seed crystal. The angle between the two primary dendritic growth segments was larger in solutions than in pure water. The variation of the angle of split with supercooling is plotted in Figures 3 and 4. It is seen from these figures that at supercoolings between 0 and about 5°C. the angle of split varied almost linearly with increasing supercooling. At larger supercoolings the angle of split varied less and at a supercooling of about 10°C. it reached, in all the solutions tested, a limiting value of about 60 angular degrees. It is also seen from Figures 3 and 4 that the variation of the angle of split with supercooling was identical in solutions of salts with a particular common cation and was larger in the solutions of sodium salts than in solutions of caesium salts.
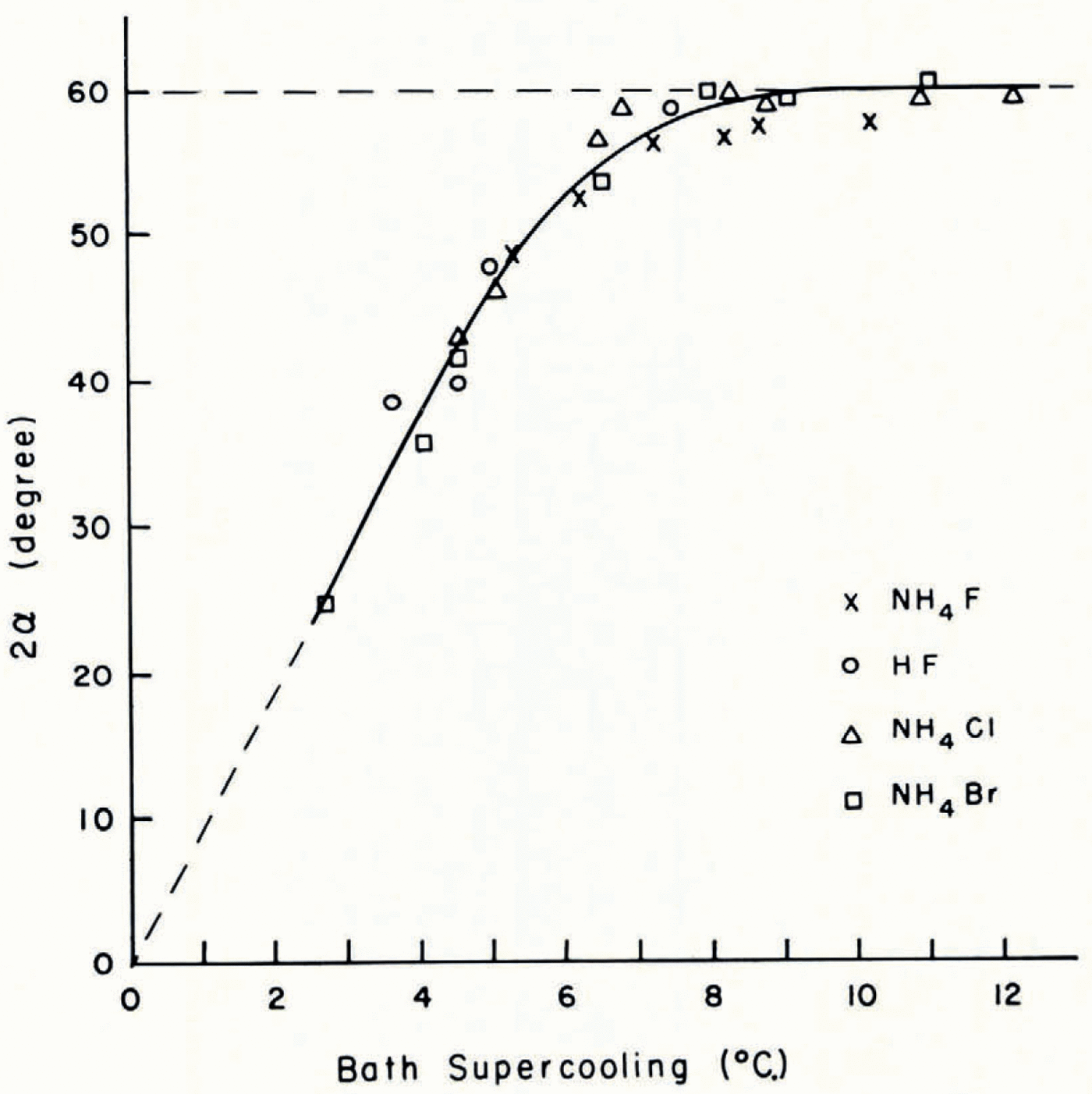
Fig. 3. Variation with supercooling of the angle 2α between the two principal growth directions of ice in 0.1 molar aqueous ammonium and 0.1 molar aqueous hydrofluoric acid solution (α = angle between principal growth direction and basal plane of seed crystal)
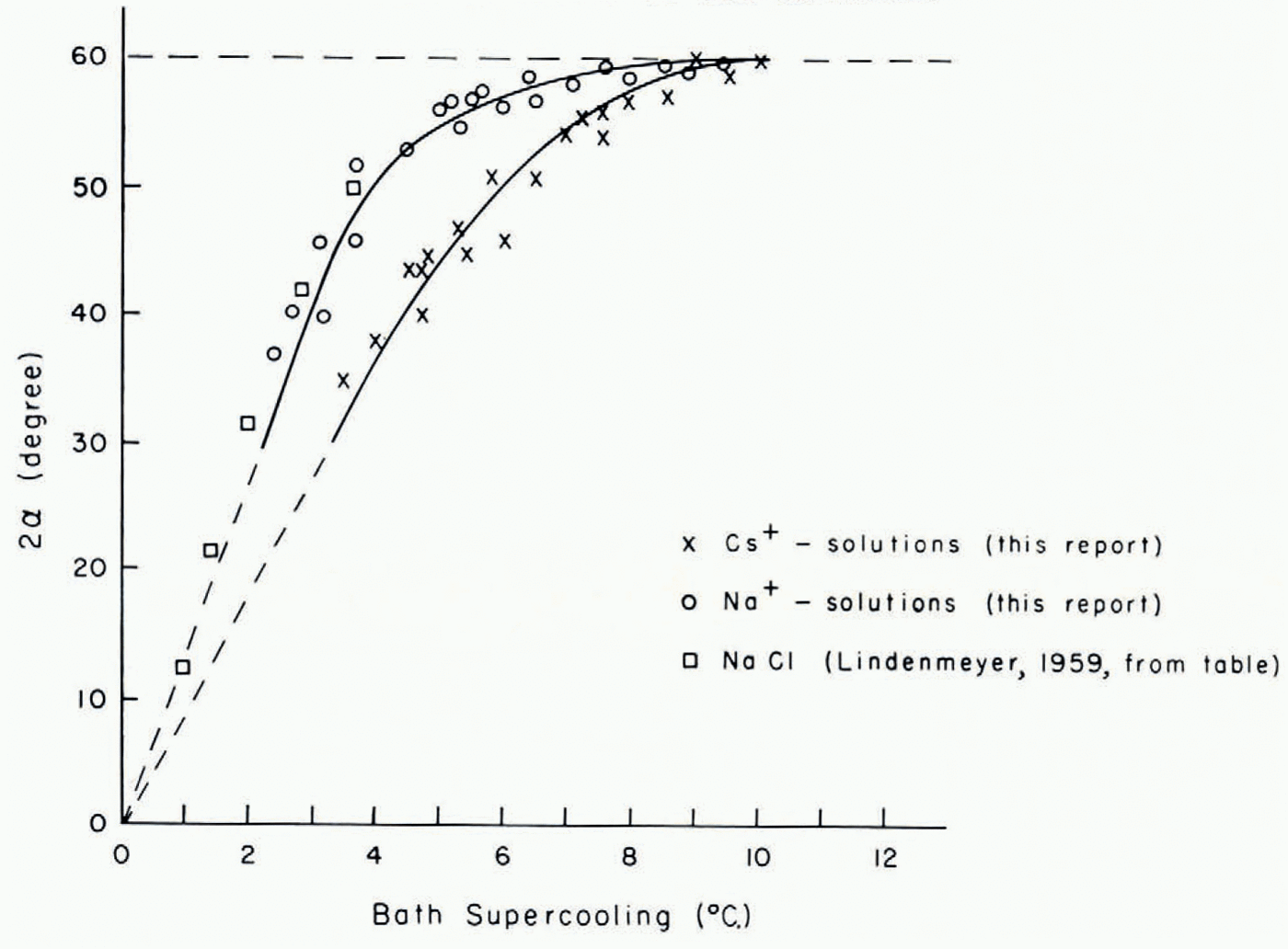
Fig. 4. Variation with supercooling of the angle 2α between the two principal growth directions of ice in various 0.1 molar aqueous sodium and caesium solutions (α = angle between principal growth direction and basal plane of seed crystal)
In the second growth regime at supercoolings larger than 5°C, a large number of higher-order split segments formed which combined with the side branches to form a three dimensional ice network of dense mesh and growth front similar to that observed in pure water at supercoolings larger than 9°C.
In solutions of concentrations between 0.1 and 1.0 mole 1.−1 the growth structures of ice became progressively complex and no special growth regimes could be observed. At concentrations between 0.5 and 1.0 mole l.−1 the growth structures often had an appearance of “flowers” or “trees” as illustrated in Figure 1q.
Discussion
As mentioned above, it was observed on ice crystals growing freely in supercooled water that at supercoolings between 0 and 1°C. the seed crystal proceeded growing undeviated into the liquid, but at supercoolings between 1 and 4°C. they split into two dendritic segments which enclosed a small angle. Based on this observation, it can be explained why in tubes at supercoolings smaller than 4°C. one or two ice dendrites were formed which grew approximately along the tube axis. The fact that in tubes at supercoolings larger than 4°C. more than two dendrites appeared whose growth direction deviated from the tube axis can be explained by the observation that at supercoolings larger than 3.5°C. freely growing ice crystals split into a number of dendritic segments which grew at a comparatively large angle to the basal plane of the seed crystal. The twisted, zigzag and screw-fashion growth which was observed after the dendrites in the tube had made contact with the tube wall can be explained on the basis of curved growth which is common for ice crystals growing on solid surfaces. The physical mechanism of curved-growth ice crystals on various substrates has been explained by Reference KnightKnight (1962) and will not be discussed here.
In order to find an explanation for the growth modes of ice crystals growing freely in supercooled water and aqueous solutions, the ice-formed structures were examined between crossed polaroids. It was found that the sheet of ice, which formed in pure water during the initial growth stage and supercoolings between 0 and 1°C., was a single crystal with its c-axis orientated parallel to that of the seed ice crystal. Drops nucleated under these conditions froze as a single crystal. The two segments of ice formed in pure water at supercoolings between 1 and 5°C. were a crystallographic entity with its c-axis orientated parallel to that of the seed crystals. Drops nucleated under these conditions also froze as single crystals. At supercoolings larger than about 5°C., multiple splitting of the seed crystal produced an ice matrix which was polycrystalline and which was able to retain considerable amounts of unfrozen liquid analogous to a sponge. Drops frozen under these conditions were polycrystalline. The polycrystallinity of this ice matrix increased with increasing supercooling. In drops of aqueous solutions the ice matrix was already polycrystalline at supercoolings as small as 2°C.
The observations showed that the freezing process of a supercooled volume of water can be divided into two phases. The first phase, during which the ice matrix is formed, occurs in a very short time and it is determined by the growth rate of ice crystals which is rapid and of the order of a few centimeters per second at a supercooling (ΔT) of a few degrees Centigrade. During this initial growth phase most of the heat is absorbed in the water so that only a fraction of about ΔT/80 of the water is turned into ice at the expense of the remainder which is warmed up towards 0°C. The second phase of the freezing process is determined by the rate of heat transfer to the environment which is, in the case of air, about two orders of magnitude slower than the first phase.
It is difficult to give a complete crystallographic explanation for the growth modes of ice observed during the first phase of the freezing process of supercooled water.
Reference HallettHallett (1964), whose results were discussed on p. 652, did not give any explanation for his observations. Reference ChalmersChalmers (1961) commented on the experimental results of Lindenmeyer (unpublished) which were discussed on p. 653. He suggested that the type of growth observed by Lindenmeyer in aqueous solutions is abnormal and unique in that it is dendritic freezing in which the growth directions are not rational directions in the crystal structure. Even though the precise crystallographic orientation of the growth segments has not been determined, the photographs presented by Reference HallettHallett (1964) and the observations documented by Figure 1a, b, l, m, l, n and l, o, and in particular by Figure 5, suggest that the growth segments are rational hexagonal dendrites. One could be tempted therefore to assume that each segment is an a-axis plane. As the adjacent segments are inclined to each other, such an assumption requires that there are two inclined a-axis planes which stem from the same primary growth direction. This would suggest a twinning mechanism. Reference Macklin and RyanMacklin and Ryan (1965) rejected the twinning mechanism as an explanation for the splitting of the seed ice crystal in supercooled water because they felt that the continuous dependence of the angle of split on the supercooling could hardly be explained on this basis, and secondly because their observations, which are discussed on p. 653, required a four-fold twinning of each a-axis.
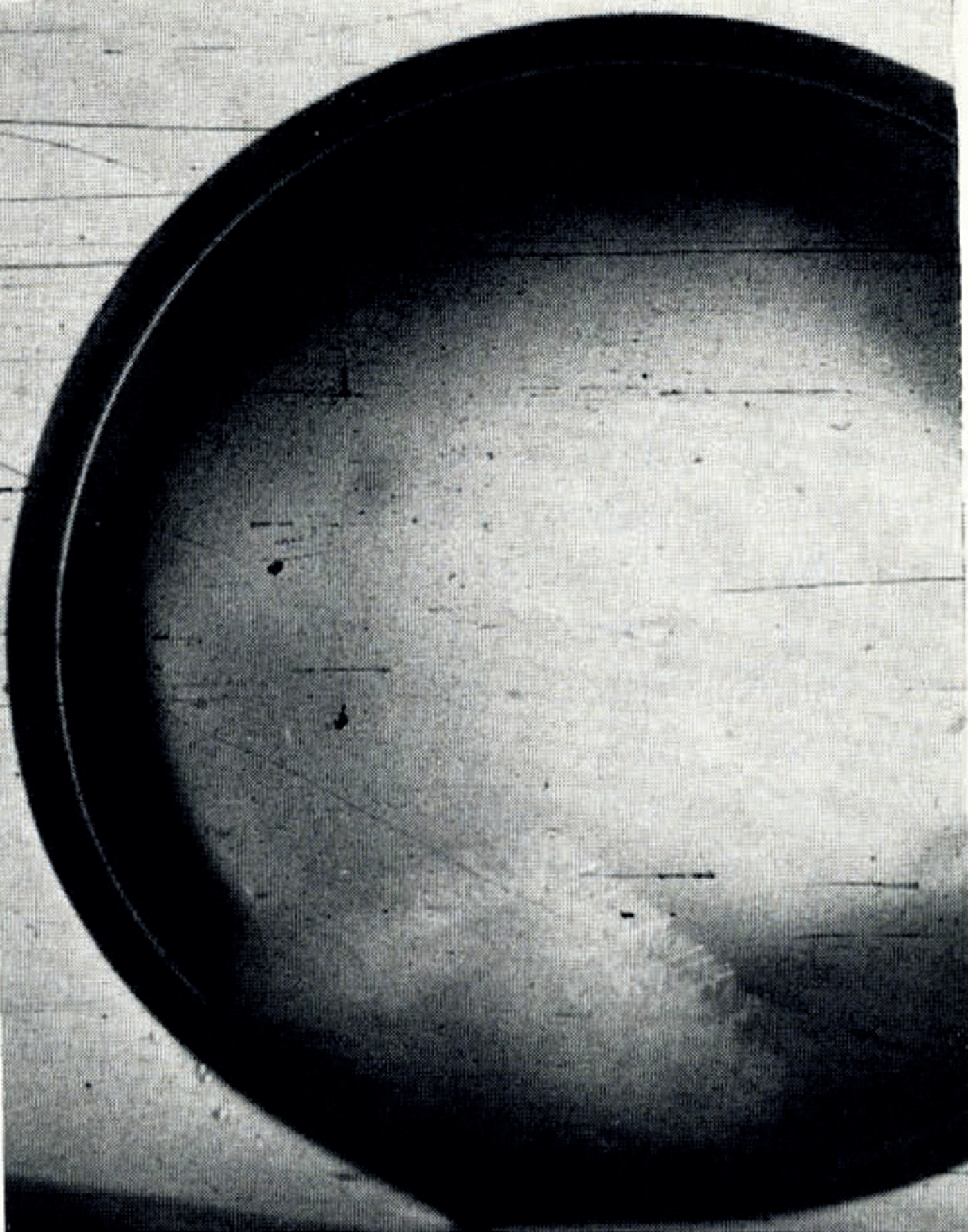
Fig. 5. Growth mode of ice in a drop of aqueous NH4Br solution (from 16 mm. motion-picture film); salt concentration 0.1 mole l.−1; supercooling 3.6°C.; drop diameter 2 cm. The crystallographic c-axis of the seed crystal is perpendicular to the photographic plane
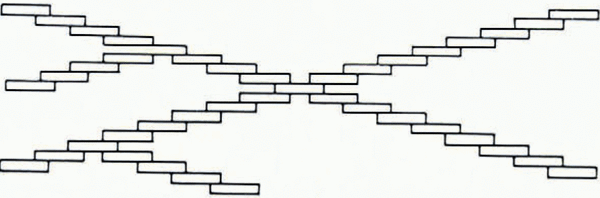
Macklin and Ryan instead proposed a stepped growth mechanism as probable for the splitting. Such a mechanism was used by Reference MasonMason and others (1963) to explain the hopper structure of ice crystals grown from vapor at moderate and high supersaturations. According to this mechanism, molecular steps on the crystal surface “bunch” together to larger steps shown in Figure 6. According to Reference Macklin and RyanMacklin and Ryan (1965), the angle of splitting is determined by the ratio between the step height and the distance between steps, that is, by the ratio of the growth velocity parallel to the c-axis direction and the velocity parallel to the basal plane.
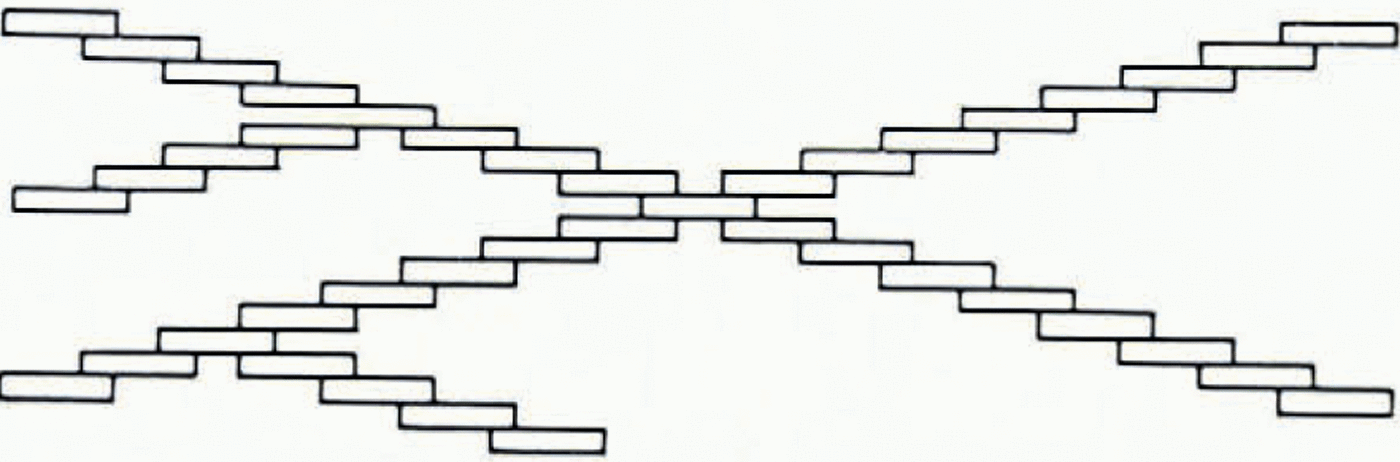
Fig. 6. Formation of ice structures in supercooled wader by the stepped growth mechanism proposed by Macklin and Ryan (adapted from Reference Macklin and RyanMacklin and Ryan (1965))
Two arguments favor the mechanism proposed by Macklin and Ryan. First, the mechanism would explain the observation that the dendritic segments caused by secondary splitting on the two primary growth segments are parallel (Fig. 1f–i, n, o). Secondly, the mechanism would require that the ice structures formed single crystals. This would be in agreement with the present observation that the ice structures formed by primary splitting of the seed crystal and secondary splitting of the two primary segments were single crystals.
There are, however, four serious arguments against the mechanism proposed by Macklin and Ryan. First, the present observations showed that the ice structures formed at supercoolings larger than 5°C. were polycrystalline. This means that a mechanism which is different from that proposed by Macklin and Ryan would have to come into operation at supercoolings larger than 5°C. However, it is difficult to see why the segments formed by multiple splitting of the seed crystal should not form in the same way as the two primary segments. Secondly, the present observations showed that the angle of split increased with increasing supercooling. This would mean in terms of the mechanisms proposed by Macklin and Ryan that the growth rate in the direction of the c-axis increases faster with increasing supercooling than the growth rate parallel to the basal plane. This is contrary to what was observed by Reference FarrarFarrar (unpublished), Reference Hillig and DoremusHillig (1958) and by Reference SperrySperry (unpublished). These investigators determined the growth rate of ice in supercooled water contained in narrow glass capillaries which were submerged in a cold bath. The growth rate was measured in the direction of the crystallographic a- and c-axes at supercoolings between 0.01 and 1.0°C. They found that in this temperature interval the growth rate in the direction of the c-axis increased less rapidly with supercooling than the growth rate in the direction of the a-axis. In contrast to this, Reference HallettHallett (1964) observed that at a supercooling of 16°C. the growth rate of ice along the c-axis differed only by a factor of 1.3 from that along the a-axis. Hallett’s observation, however, is based only on a single measurement and it disagrees sharply with the observation documented by Figure 1j, which shows that even at supercoolings as large as 9°C. the ice matrix formed during the initial growth phase consists of a very large number of thin dendrites. Thirdly, the present observations showed that the angle of split is larger in aqueous solutions than in pure water. This would mean in terms of the mechanism proposed by Reference Macklin and RyanMacklin and Ryan (1965) that the rate of formation of new growth layers in the direction of the c-axis is less inhibited by dissolved salts than the growth rate in the direction of the basal plane. In contrast to this, Farrar (unpublished) and Sperry (unpublished) observed that the growth rate of ice along the c-axis in aqueous solutions is very strongly reduced at supercoolings smaller than 1°C., while the growth rate of ice along the a-axis at supercoolings smaller than 1°C. is not affected by dissolved salts even in solutions of concentrations as high as 1 mole 1−1 Also, the present observations showed that the growth rate of ice along the a-axis in alkali halide solutions of concentrations as large as 10−1 mole 1.−1 was not reduced significantly below that of ice in pure water as long as the supercooling was smaller than 4°C. It must therefore be concluded that the growth rate of ice in aqueous solutions is more strongly reduced in the c-axis direction than in the a-axis direction. Fourthly, it was observed that the angle of split reached a limiting value, which was 45 angular degrees in pure water and 60 angular degrees in aqueous solutions independent of the salt dissolved. It is difficult to see how this result could be explained in terms of the mechanism proposed by Reference Macklin and RyanMacklin and Ryan (1965).
Even though there is little doubt that growth of ice crystals in supercooled water and aqueous solutions takes place by a stepped growth mechanism at least to supercoolings as large as 12°C., the discussion given above suggests that it is difficult to make such a growth mechanism responsible for the splitting of the seed crystal and for the magnitude of the angle between the split elements. However, new, accurate crystallographic investigations have to be carried out in order to give a complete and satisfactory explanation for the growth mechanism of ice in supercooled water and aqueous solutions.
Acknowledgement
I am much indebted to Professor M. Neiburger for his continued encouragement and his interest in the research presented in this paper.