Introduction
Snow has the unique characteristic of filtering and collecting aerosols from the atmosphere and holds the potential to record the chemical composition changes of the atmosphere over time. Therefore, study of the chemical compositions of fresh-snow samples from glaciers can reveal information on atmospheric components, sources, transport pathways and background atmospheric aerosol concentrations (Reference Mayewski, Lyons and AhmadMayewski and others, 1983; Reference ShawShaw, 1988). In addition, the understanding of present relationships of aerosols and moisture sources is crucial in interpreting temporally longer glaciochemical records such as ice cores, which are proven to be an excellent tool to investigate the atmospheric composition of the past (Reference Mayewski, Lyons, Ahmad, Smith and PourchetMayewski and others, 1984; Reference Legrand and MayewskiLegrand and Mayewski, 1997). Glaciochemical studies in southern South America have been limited to a few studies in Patagonia (Reference YamadaYamada, 1987; Reference Aristarain and DelmasAristarain and Delmas, 1993; Reference ShiraiwaShiraiwa and others, 2002; Reference Schwikowski, Brütsch, Casassa and RiveraSchwikowski and others, 2006; Reference VimeuxVimeux and others, 2008) and no data are available for the Cordillera Darwin, the southernmost mountain range in South America and the glaciated region closest to Antarctica. As with most Andean and Patagonian glaciers, Tierra del Fuego is remote from industrial centers; however, local cities/towns, logging activities and marine traffic may expose glaciers to measurable amounts of pollutants. We present results of fresh-snow concentrations of stable isotopes, major soluble ions and major and trace elements during a 17 day sampling campaign in the late austral summer (February–March) of 2006. The purpose of this study was to retrieve chemical concentrations in fresh snow and to evaluate inter-species relationships (e.g. marine, crustal and possible anthropogenic influences) along with meteorological mechanisms. Statistical analysis of the chemistry time series and on-site barometric pressure measurements, examination of US National Oceanic and Atmospheric Administration (NOAA) Hysplit 7 day back trajectories, and preliminary regional glaciochemical record comparisons are presented.
Study Area
The research site was located on Glaciar Marinelli (54°38.878′ S, 69°43.206′ W; 1823 m a.s.l.; 142 km2) in the Cordillera Darwin in the southwestern region of Tierra Del Fuego, Chile (Fig. 1). Glaciar Marinelli is the largest glacier in the Cordillera Darwin and is reported to have receded 5 km between 1960 and 1993 (Reference Holmlund and FuenzalidaHolmlund and Fuenzalida, 1995). Recent observations from the summit of Glaciar Marinelli during the snow-sampling campaign suggest that the recession is progressing. The Cordillera Darwin is close to large moisture sources, the two largest being the Pacific Ocean (∼100 km to the west) and the Atlantic Ocean (∼150 km to the east).

Fig. 1. Location map (Google Maps image). Glaciers and stations labeled contain available snow- and ice-chemistry data (see references in text).
As a result of limited land masses in the southern Pacific and a strong temperature gradient between the Antarctic continent and the Southern Hemispheric subtropics, the Cordillera Darwin is dominated by strong year-round westerlies (Reference Miller and SchwerdtfegerMiller, 1976). Evaluation of the Tierra del Fuego region with US National Centers for Environmental Prediction (NCEP)/US National Center for Atmospheric Research (NCAR) reanalysis (Reference KalnayKalnay and others, 1996) shows the surface-level scalar wind velocities reaching maximums in the austral spring (September–November) and austral summer (December–February). Lowest wind velocities are experienced during the austral winter (June–August). Mean annual scalar wind velocities are ∼8 m s−1. Monthly NCEP/ NCAR surface precipitation data (covering 1960–2000) show that Tierra del Fuego receives steady precipitation throughout the year, but the greatest precipitation occurs during spring and winter.
Methodology
Snow sampling
The fresh surface snow study was conducted in the accumulation zone of Glaciar Marinelli from 24 February to 12 March 2006. Samples were collected in the morning, at midday and in the evening, upwind of camp on a gently sloped glacier section away from valley walls and crevasse fields. Sample-collection times are presented in Table 1. Snowfall persisted almost throughout the sampling campaign. Each sample was collected between (6 samples) or during (20 samples) snowfall events. Samples were collected using a plastic spade pre-cleaned with deionized (DI) water (>18.2 MΩ) by a researcher wearing a non-particulating suit, face mask and wrist-length polypropylene (PP) gloves. To assure that each sample represented the most recent snowfall, only the top centimeter of surface snow was collected and placed into Teflon bags. The Teflon bags were subsequently stored inside Whirl-Pak® bags. Twenty-six snow samples were collected and poured into high-density polyethylene (HDPE) vials for analysis of stable-isotope ratios (δ18O) and into pre-cleaned (with DI water) polypropylene (PP) vials for analysis of soluble ion concentrations (Na+, K+, Ca2+, Mg2+, Cl−, NO3 −, SO4 2−, MS− (methane sulfonate)). Major- and trace-element (Na, Mg, Al, Fe, Ca, Sr, Cd, Cs, Ba, La, Ce, Pr, Dy, Ho, Er, Bi, U, Ti, V, Cr, Mn) vial-cleaning procedures are presented by Reference Osterberg, Handley, Sneed, Mayewski and KreutzOsterberg and others (2006). The samples were analyzed for stable isotopes, major soluble ions and trace elements at the Climate Change Institute, University of Maine, using a Micromass Isoprime ion chromatograph, a VG/Micromass SIRA, a Dionex DX-500 ion chromatograph and a Thermo-Finnigan Element 2 inductively coupled plasma sector field mass spectrometer (ICP-SMS) respectively. The ICP-SMS is coupled with a microflow nebulizer/desolvation introduction system to reduce potential spectroscopic interferences (Reference Field and SherrellField and Sherrell, 2003; Reference GabrielliGabrielli and others, 2006). ICPSMS samples were acidified to 1% with double-distilled HNO3 under a class 100 High Efficiency Particle Air (HEPA) clean bench and allowed to react with the acid for approximately 1 week before being frozen. Samples were melted at room temperature approximately 24 hours prior to analysis. A summary of Teflon bag blank values and calculated detection limits (3σ) is presented in Table 2. All major soluble-ion and element-sample concentrations were more than three times Teflon bag detection limits (TBDL), except for the minimum value of Cr and Pr in two of the samples. The minimum value of Cr was less than TBDL, so the detection limit was used for statistical calculations. However, the minimum value of Pr was approximately three times the TBDL and therefore was left unchanged.
Table 1. Sample-collection times and co-registered temperature and pressure measurements

Table 2. Statistical summary of stable isotopes (‰ Standard Mean Ocean Water (SMOW)), major soluble ion (μg L−1) and major trace element (ng L−1) concentrations determined at Glaciar Marinelli (24 February–12 March 2006)

Observed meteorology
During the sampling sessions, temperature and barometric pressure were recorded using a Kestrel® 2500 Pocket Wind Meter (Table 1). Daily measurements of temperature, observations of mixed rain–snow precipitation, and melt layers detected in snow pits suggested that temperatures above freezing are present during the summer. The on-site mean temperature was −0.96 ± 1.95°C. NCEP/NCAR reanalysis data for the sampling site between 24 February and 13 March 2006 at the 850 hPa level revealed mean air temperature of 0–1°C. During the 3 week field campaign, heavy snowfall was observed. A 3 m long accumulation/ velocity stake revealed a ∼1.2 m snow accumulation over a 15 day period. On-site mean barometric pressure was 790.45 ± 8.17 hPa.
Results and Discussion
Concentrations and statistical analysis of major soluble ions
Statistical summaries of soluble ion concentrations are presented in Table 2. The total median concentration load was 5481 μg L−1. Based on concentration, major soluble ions can be categorized into two groups: (1) high concentrations: Cl−, Na+ and SO4 2−representing 46.2%, 25.8% and 15.8% of total ion concentrations respectively; and (2) low concentrations: K+, Ca 2+, MS−, Mg2+ and NO3 − representing 3.3%, 2.9%, 2.0%, 1.9% and 2.0% of total ion concentration respectively. The dominant presence of the major sea-salt components Cl− and Na+ reflects the proximity of Glaciar Marinelli to the Pacific Ocean. The mean Cl−/Na+ ratio was 1.17, very close to the sea-water ratio of 1.16.
In order to help determine the sea-salt (ss) and non-seasalt (nss) contributions of the major soluble ion load, nonsea-salt and sea-salt ratios were calculated using
where X is the soluble ion concentration of snow sample (μg L−1), k is [X]/[Na+ standard sea water] and nssX is the non-seasalt X concentration of the snow sample (μg L−1).
Na+ was used as the reference species and is therefore assumed to originate completely from sea water. Sea-water ratios are from Reference HollandHolland (1978). Calculations indicate that 99% of Cl− and Mg2+ is derived from sea water. This is not surprising given the proximity of the site to the Pacific Ocean. Concentrations of Ca2+, K+ and SO4 2−, however, were suggested to have additional nss sources accounting for 50%, 61% and 52% of each ion respectively. SO4 2− represents 15.8% of total ion concentrations; this high concentration of ssSO4 2 and nssSO4 2 is most likely a result of sea spray and biogenic productivity from the nearby ocean. Additional sources of nssSO4 2− may originate from dust, nearby volcanic emissions and local anthropogenic activity.
Empirical orthogonal function (EOF) analysis was conducted on the major soluble ion suite (Na+, nssK, Cl−, Mg2+, nssCa2+, nssK+ and nssSO4 2−) to investigate common variability and inter-species relationships (Table 3). EOF1 accounts for ∼43% of the total variance in the soluble ions. Na+, Cl− and Mg2+ are strongly loaded on EOF1 (∼95%), representing the influx of sea-water source ions. EOF2 represents ∼26% of the total variance and is strongly loaded positively by nssK+ (∼60%) and nssCa2+ (∼64%), indicating probable dust species. nssSO4 2− does not load strongly on EOF2 and, as suggested earlier by marine weight calculations, does not appearto have a strong crustal source. Instead, nssSO4 2− is primarily grouped with NO3 − on EOF3, which represents ∼10% of total ion variance, at 32% and 67% respectively. Both SO4 2−and NO3 − are known anthropogenic pollutants and their common variance may suggest that nearby anthropogenic activities are impacting snow chemistry. MS−, an atmospheric oxidation product of dimethyl sulfide originating from biogenic marine sources, is primarily (40%) loaded on EOF4 (∼7% of total ion variance).
Table 3. EOF analysis of major soluble ions at Glaciar Marinelli

Concentrations and statistical analysis of major and trace elements
Statistical summaries of major and trace element concentrations are presented in Table 2. Based on concentrations, trace elements can be categorized into three groups: (1) high concentrations: Na represents 74.7% of total element concentrations; (2) intermediate concentrations: Mg, Al, Ca and Fe represent 10.1%, 6.5%, 6.1% and 2.4% respectively; (3) low concentrations: the remaining elements combined represent 0.2%. Na and Mg, as with the soluble ions, are assumed to completely originate from sea water. Ca showed the same nss and ss percentages as Ca2+. Al and Fe are assumed to reflect nss concentrations because of their minute concentrations in sea water.
Enrichment factors (EF) were calculated to provide compositional major and trace element comparisons between snow samples and average upper-crust values (Fig. 2.). EFupper crust values were calculated using:
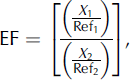
where EF is the enrichment factor of snow-sample to upper-crust values, X 1 is the concentration of element in the snow sample (ng L−1), X 2 is the concentration of element in the upper crust (ng L−1), Ref1 is the concentration of the reference element in the snow sample (ng L−1) and Ref2 is the concentration of reference element in the upper crust (ng L−1).

Fig. 2. Median enrichment factors for the measured elements.
Al, Fe, Ba and La were used as the reference elements to reduce any potential bias from one upper-crust element (Reference PlanchonPlanchon and others, 2002; Reference BarbanteBarbante and others, 2003; Reference OsterbergOsterberg and others, 2008). The trace element concentrations for the average upper crust are from Reference WedepohlWedepohl (1995). All reference elements produced similar EF values. The medians of reference element (Al, Fe, Ba and La) EF values are presented in Figure 2. Due to dust source areas that may have element concentrations greater than mean upper-crust values, researchers have suggested that EFupper crust values between +10 and −10 are indicative of dominant inputs from rock/soil dust (Reference BarbanteBarbante and others, 2003). EF values >10 may suggest non-crustal, notably anthropogenic influences. The proximity of major sea-water sources must be considered when evaluating the trace element EF values. The majority of the trace elements display EF values very close to upper-crust values between 1 and 3. The high EF values for Na and Mg are assumed to be products of dominant sea-water contributions. Median EF values for Cr are slightly above 10 (18.34) and may suggest that anthropogenic pollutants are reaching the site. The greatest median EF values are very high and exhibited by Bi and Cd reporting values of 719.75 and 210.96 respectively. These high values are most likely attributable to nearby cities/towns (e.g. Punta Arenas), local logging or perhaps local marine transportation. Another potential source of the enriched Bi and Cd may be local volcanism (i.e. degassing) (Reference RosmanRosman and others, 1998; Reference Matsumoto and HinkleyMatsumoto and Hinkley, 2001; Reference VallelongaVallelonga and others, 2004). High EF values of Cd and Bi (several hundred times crustal compositions) were reported by Reference Mishra, Kim, Hong and LeeMishra and others (2004) in an aerosol study at King Sejong Station on King George Island (∼1000 km southeast of Glaciar Marinelli; Fig. 1) and attributed to local anthropogenic emissions and/or long-range transported anthropogenic pollutants. Several sites in Antarctica have also reported levels of heavy metal anthropogenic pollutants (Reference Boutron, Candelone and HongBoutron and others, 1994; Reference PlanchonPlanchon and others, 2002; Reference VallelongaVallelonga and others, 2004).
EOF analysis was also conducted on the major and trace element suite to investigate common variability and inter-species relationships (Table 4). EOF1 accounts for ∼53% of the total variance in the trace elements and is heavily loaded with the elements Fe, Al, Mn, Cs, Ba, Bi, V, Ti and the rare-earth elements (REE) Ho and Er (79–89%). Based on the elemental composition and EF calculations, EOF1 appears to represent a dust source signal. Bi, previously mentioned as a potential anthropogenic species, may have transport pathways similar to those of the primary dust aerosol input. EOF2 represents ∼16% of the total variance and is strongly loaded by known sea-salt elements Na (∼57%), Mg (∼48%) and Sr (47%) (Reference Ikegawa, Kimuva, Honda, Makita, Fujii and ItokawaIkegawa and others, 1997). Ca is primarily divided between EOF2 (∼36%) and EOF1 (∼30%), suggesting that Ca inputs are from both marine and dust sources. EOF3 (∼15% of the total variance) is strongly loaded by REEs (La (∼69%), Ce (∼49%) and Pr (∼63%)), which are expected to derive from a crustal source and may represent a secondary dust input. Cd is also strongly loaded on EOF3, but it is biased by one particular Cd concentration that is an order of magnitude above the median Cd value and therefore may possibly represent contamination rather than an anthropogenic source. EOF4 represents ∼6% of the total variance and is loaded by Cr (∼43%) and could potentially be a crustal input or anthropogenic as EF values suggest.
Table 4. EOF analysis of major and trace elements at Glaciar Marinelli

Given the limited time period of the fresh-snow surface study, it is not possible to assess seasonal variations of trace elements at Glaciar Marinelli. However, an atmospheric aerosol study reported by Reference Mishra, Kim, Hong and LeeMishra and others (2004) at King Sejong Station, subject to regional atmospheric circulation similar to that at the Cordillera Darwin, may offer a comparable example of relative summer concentrations of trace elements, as well as possibly suggest the seasonality of trace elements that might be exhibited at Glaciar Marinelli. Of the similar trace elements analyzed, it was observed that on KGI, Bi, Cd and Cs displayed maximum concentrations during the summer. These high levels of Bi and Cd, previously mentioned as potential anthropogenic species, correspond to increased human activity during the summer season on KGI. However, as reported by Reference IriondoIriondo (2000) and Reference Mishra, Kim, Hong and LeeMishra and others (2004), summer winds are predominately from the northwest and may transport crustal and anthropogenic elements from South America. Al, Ba, Cr and V showed maximum concentrations during the autumn, and Sr peaked in the spring. Although, the Cordillera Darwin and KGI share similar regional meteorology, marked differences may be present in the seasonal variations of trace elements as a result of Glaciar Marinelli’s proximity to larger crustal (ice-free land) and anthropogenic sources, particle scavenging during transport, and the presence of sea ice.
δ18O, barometric pressure and air-mass trajectories
δ18O and on-site barometric pressure are presented in Figure 3. Time series are not continuous. On 1 and 4 March, samples were not collected. δ18O values (statistical summary in Table 2) vary steadily over the 17 day period, with three peaks in less depleted δ18O values and three troughs in more depleted δ18O values. Examination of these δ18O trends in relation to recorded on-site observations of barometric pressure suggests a possible connection. The linear correlation coefficient between δ18O and pressure is 0.60 (p < 0.007, n = 18). Stable-isotope ratios are controlled by air temperature, amount of precipitation (amount effect) and the air-mass transport from source regions. In terms of pressure, which can indicate storm conditions (i.e. intensity), the positive correlation with δ18O may suggest that stronger storm conditions/lower pressure coincided with greater precipitation, resulting in more depleted values of δ18O (amount effect). On-site air-temperature observations revealed no relationship with δ18O (r = 0.06), and on-site precipitation measurements were not conducted.

Fig. 3. Temporal trends of δ18O and pressure at Glaciar Marinelli. Discontinuities in trend lines indicate days with no collected samples.
Seven-day Hysplit backward trajectory analysis (NOAA, http://www.arl.noaa.gov/ready/open/hysplit4.html) indicated that air-mass movements during the snow sample campaign (24 February–12 March 2006) were primarily from the northwest, but also from the southwest and west, suggesting that the moisture source and majority of chemistry species originated from the Pacific Ocean and the Drake Passage (Fig. 4). The trajectories ranged from ∼30° S to ∼80° S, reaching regions such as Antarctica, the Weddell Sea and New Zealand. Based on the air-mass trajectories and far distances to land masses, dust and anthropogenic species are probably local, but long-range transport of dust and pollution from arid and industrial sources (e.g. Australia) cannot be excluded.

Fig. 4. Hysplit 7 day backward trajectories (24 February–12 March 2006) impacting the sampling site (Google Earth image).
Regional major-ion records
To make additional interpretations of the snow chemistry data from Glaciar Marinelli, major-ion concentrations are compared to results reported at nearby sites, ranging from northern sites (e.g. Cerro Tapado (∼30° S, 69° W; 5536 m a.s.l.)) in the central Andes to southern sites on KGI (62°07′ S, 58°37′ W; 575–690 m a.s.l.; Fig. 1). It is important to recognize that seasonal variations of chemistry at Glaciar Marinelli are not established, so comparisons are preliminary. Glaciar Marinelli mean surface snow concentrations and available annual mean concentrations from snow-pit and ice-core datasets are presented in Table 5. Based on marine weight calculations at most of the sites, Na+ and Cl− concentrations are primarily sourced from sea water. The calculation was not conducted for Monte San Valentín (MSV) and Tyndall glaciers because annual mean concentrations have not been published; however, Na+ and Cl− concentrations were presumed to be dominantly from marine sources (Reference ShiraiwaShiraiwa and others, 2002; Reference VimeuxVimeux and others, 2008). At Mercedario, Na+ concentrations are higher than Cl−, yielding a Cl−: Na+ ratio of 0.82, significantly lower than the average sea-water ratio of 1.16 and suggesting an additional input of Na+ from a nss source. The contribution of ss versus nss ions is used in this study to classify sites as either marine- or crustal-source dominated. The more southerly sites (Glaciar Marinelli, Gorra Blanca Norte (GBN) and Glaciar Perito Moreno (GPM)) are dominated by marine inputs (ssNa+ and ssCl−), while the more northerly sites (Mercedario, Plomo and Tapado) are more influenced by crustal inputs (nssCa2+ and nssSO4 2−). This is not surprising since the northern sites are further inland and located in arid regions where dust sources are more abundant. Ca2+ and SO4 2−are the two most abundant ions at Mercedario, Plomo and Tapado. Marine weight calculations suggest nssCa represents >98% of the Ca 2+, and nssSO4 2− >97% of the SO4 2−, at these sites. The high values of SO4 2−were also suggested to be from a local anthropogenic emission source (e.g. Santiago) (Reference Bolius, Schwikowski, Jenk, Gäggeler, Casassa and RiveraBolius and others, 2006). Seasonality at Mercedario shows clear increases in all major ions during the summer. The enrichment of summer chemistry was attributed to the intensity of vertical mixing in the atmosphere, and sublimation at the site.
Table 5. Annual mean major soluble ion concentrations (μEq L−1)

The near-coastal location and distance from major dust-source regions of the southern (South American) sites explain the dominant marine influence and are reflected by high concentrations of Na+ and Cl− relative to predominately crustal ions (e.g. Ca2+). The differences in mean Na+ and Cl− concentrations between the southern South American sites may be due to a combination of elevation, distance from the Pacific Ocean and air-mass trajectories. MSV, located at the highest elevation (3747 m), displays the lowest Na+ concentration, ∼0.2–1.0 μEq L−1 (estimated from 1989 Na+ depth/age graph; Reference VimeuxVimeux and others, 2008). The southern site’s Na+ and Cl− concentrations appear inversely related to elevation. Glaciar Tyndall, located at the lowest elevation (1756 m), has the highest concentrations of Na+ and Cl−. Reference ShiraiwaShiraiwa and others (2002) state that the major ions (Na+, Cl− and NO3 −) at Glaciar Tyndall are an order of magnitude higher than those reported by Reference Aristarain and DelmasAristarain and Delmas (1993), but the rapid decrease in ions over the top 4 m by meltwater elution precludes the estimate of annual concentrations. The reason mean Na+ and Cl− concentrations are higher at Glaciar Marinelli than at other Patagonian sites (except Glaciar Tyndall) may not be elevation or marine source distance, but particular atmospheric conditions during the 17 day field campaign. GBN, a nearby site that shares similar regional atmospheric circulation, displays large peaks in summer concentrations of ss species (Na+, Cl− and Mg2+). The increases in ss concentrations were attributed to the high summer wind speeds that promote sea-spray formation. Extrapolation of this seasonal signal to Glaciar Marinelli would suggest that above-annual-mean ss concentrations were more likely deposited. Further south, the KGI sites display extremely high annual concentration means of Cl− and SO4 2−compared with the South American sites. The high values of marine species can be attributed to the low elevation of the sites and close proximity to sea water. nssSO4 2− concentrations on KGI are attributed mainly to biogenic dimethyl sulfate (DMS) and possibly volcanic sources (Reference SimõesSimões and others, 2004).
Comparisons between NO3 − concentrations show marked differences between northern and southern sites of South America. Mean NO3 − concentrations are an order of magnitude greater at northern than at southern sites, most likely due to more abundant dust source locations. In addition, Reference Bolius, Schwikowski, Jenk, Gäggeler, Casassa and RiveraBolius and others (2006) suggested that high NO3 − levels at Mercedario and Cerro Tapado may be due to local anthropogenic emission sources from cities such as Santiago, Chile. The southern sites, GBN and GPM, are reported to have lower NO3 − concentrations than pre-industrial medians from a core from the European Alps (Reference Schwikowski, Döscher, Gäggeler and SchottererSchwikowski and others, 1999). The low NO3 − concentrations present at Glaciar Marinelli are similar to Reference Schwikowski, Döscher, Gäggeler and SchottererSchwikowski and others (1999) and also reflect the low anthropogenic emissions in southern South American regions (e.g. Patagonia and Tierra del Fuego). Although, annual mean NO3 − values are not available, a depth/time graph revealed similarly low concentrations at MSV (Reference VimeuxVimeux and others, 2008). Because pre-industrial NO3 − concentrations have not been assessed in the region and NO3 − contribution from dust sources is unclear, it is difficult to evaluate anthropogenic impacts via concentration levels alone. However, previously mentioned EF calculations of Glaciar Marinelli chemistry suggest possible anthropogenic influences. KGI sites show varying NO3 − concentrations, with low levels (below detection limit) at the flat ice divide of Lange Glacier (62°07′ S, 58°37′ W; 690 m a.s.l.), similar to southern South American sites, but, very high NO3 − concentrations at the lower-elevation and more near-coastal sites (Drake and Ezurra glaciers) (Table 3). The higher concentrations may be explained by local rock sources, local emission from research stations, or perhaps post-depositional processes (e.g. superficial melting, percolation and refreezing) which can alter ion content. KGI summer temperatures at sea level are frequently above 0°C (Reference Ferron, Simões, Aquino and SetzerFerron and others, 2004). Major-ion concentrations from Lange Glacier were considered unaltered (top 2.7 m) and representative of the original precipitation, thereby providing background chemical compositions (Reference SimõesSimões and others, 2004).
Summary
This is the first report on fresh-snow chemistry (stable isotopes, major ions and trace elements) from Glaciar Marinelli. Data interpretations suggest that snow chemistry is dominantly loaded by marine species (Cl−, Na+ and ssSO4 2−). Na+ and Cl− account for ∼70% of the total ion load, and Na accounts for ∼75% of total element loading. Contributions of crustal chemistry appear to be very low; estimated nssCa only represents ∼1% of the total ion load, and nssCa, Al, and Fe represent approximately 6%, 6% and 2% of the total trace element load respectively. EOF analysis may suggest two possible dust sources, one primarily represented by Al and Fe and another by REE (La, Ce, Pr). EF calculations of trace elements suggest the majority of elements are within average upper-crustal ratios. Cr shows moderate median enrichment values at ∼20, but major median enrichments of Bi and Cd (hundreds of times) suggest possible anthropogenic sources. Anthropogenic inputs are also suggested by EOF ion grouping of nssSO4 2− with NO3 −. Pollutant sources may originate from local cities/ towns (e.g. Punta Arenas), logging activities and marine traffic. The linear relationship between δ18O and on-site barometric pressure (r = 0.60, p<0.007,n = 18) may suggest that stronger storm conditions coincided with greater precipitation (amount effect), resulting in more depleted values of δ18O. During the sampling period, back-trajectory analysis shows air masses coming primarily from the west and southwest. The dominance of marine species and concentrations of NO3 − are similar to the findings of previous studies in Patagonia. Additional temporal and spatial glaciochemical sampling of Glaciar Marinelli is necessary to determine seasonal variations of snow chemistry. This study, however, provides a preliminary assessment of snow chemistry from the southernmost glaciated region outside Antarctica.
Acknowledgements
This research was funded by US National Oceanic and Atmospheric Administration grant NOAA NA06OAR4310206. We especially thank Centro de Estudios Científicos researchers J. Wendt and A. Wendt for logistical help, and DAP for helicopter support.











