Article contents
Sharp transition between coalescence and non-coalescence of sessile drops
Published online by Cambridge University Press: 04 March 2014
Abstract
Unexpectedly, under certain conditions, sessile drops from different but completely miscible liquids do not always coalesce instantaneously upon contact: the drop bodies remain separated in a temporary state of non-coalescence, connected through a thin liquid bridge. Here we investigate the transition between the states of instantaneous coalescence and temporary non-coalescence. Experiments reveal that it is barely influenced by viscosities and absolute surface tensions. The main system control parameters for the transition are the arithmetic means of the three-phase angles, 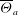 $\overline{\Theta }_{a}$, and the surface tension differences
$\overline{\Theta }_{a}$, and the surface tension differences 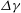 $\Delta \gamma $ between the two liquids. These relevant parameters can be combined into a single system parameter, a specific Marangoni number
$\Delta \gamma $ between the two liquids. These relevant parameters can be combined into a single system parameter, a specific Marangoni number 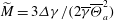 $\widetilde{M}=3\Delta \gamma /(2\overline{\gamma }\overline{\Theta }_{a}^2)$. This
$\widetilde{M}=3\Delta \gamma /(2\overline{\gamma }\overline{\Theta }_{a}^2)$. This 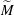 $\widetilde{M}$ universally characterizes the coalescence transition behaviour as a function of both the physicochemical liquid properties and the shape of the liquid body in the contact region. The transition occurs at a certain threshold value
$\widetilde{M}$ universally characterizes the coalescence transition behaviour as a function of both the physicochemical liquid properties and the shape of the liquid body in the contact region. The transition occurs at a certain threshold value 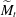 $\widetilde{M}_t$ and is sharp within the experimental resolution. The experimentally observed threshold value of
$\widetilde{M}_t$ and is sharp within the experimental resolution. The experimentally observed threshold value of 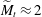 $\widetilde{M}_t\approx 2$ agrees quantitatively with values obtained by simulations assuming realistic material parameters. The simulations indicate that the absolute value of
$\widetilde{M}_t\approx 2$ agrees quantitatively with values obtained by simulations assuming realistic material parameters. The simulations indicate that the absolute value of 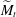 $\widetilde{M}_t$ very weakly depends on the molecular diffusivity.
$\widetilde{M}_t$ very weakly depends on the molecular diffusivity.
- Type
- Rapids
- Information
- Copyright
- © 2014 Cambridge University Press
References
Karpitschka and Riegler supplementary movie
One drop of Tetradecane and one drop of Pentadecane spread on the same substrate. They contact each other at three phase angles above the critical value; Coalescence is (initially) immediate.
Karpitschka and Riegler supplementary movie
One drop of Tetradecane and one drop of Pentadecane spread on the same substrate. They contact each other at Three phase angles below the critical value; Coalescence is suppressed.
Karpitschka and Riegler supplementary movie
One drop of Tetradecane and one drop of Hexadecane spread on the same substrate. They contact each other at three phase angles above the critical value; Coalescence is (initially) immediate.
- 30
- Cited by


