Introduction
Birth weight involves the risk of birth complications,Reference Koyanagi, Zhang and Dagvadorj 1 neonatal complications,Reference McIntire, Bloom, Casey and Leveno 2 postnatal growthReference Mei, Grummer-Strawn, Thompson and Dietz 3 and non-communicable diseases (NCDs) in offspring later in life.Reference Barker, Hales and Fall 4 – Reference Sugihara, Sasaki and Amemiya 6 Thus, the prenatal period may be an important stage for determining an infant’s phenotype in terms of parental genetics, epigenetics or phenotypic parameters. Understanding the factors influencing birth weight may offer insight into the evolutionary processes affecting early development.
Parental height and parental obesity are known to be two of several factors associated with birth weight. Previous studies have consistently shown that maternal body mass index (BMI) is positively associated with infant birth weight,Reference Regnault, Botton and Forhan 7 – Reference Knight, Shields and Hill 9 suggesting that the maternal intrauterine environment plays a substantial role in determining birth weight. On the other hand, studies of paternal BMI and its influence on birth weight show contradictory results. Miletic et al. showed a significant association between paternal BMI and birth weight.Reference Miletic, Stoini and Mikulandra 10 However, several other studies have reported no significant influence,Reference Morrison, Williams, Najman and Andersen 11 – Reference Kuzawa and Eisenberg 17 indicating that paternal height has a stronger impact on fetal growth than paternal BMI.Reference Morrison, Williams, Najman and Andersen 11 – Reference Knight, Shields and Turner 13 , Reference Griffiths, Dezateux and Cole 15
In a Japanese population, detailed associations between paternal height or BMI and infant birth weight remain unclear, particularly regarding the prevalence of small for gestational age (SGA), appropriate for gestational age (AGA) and large for gestational age (LGA). The Japanese Ministry of the Environment launched a large-scale epidemiological research project called the Japan Environment and Children’s Study (JECS) in January 2011.Reference Kawamoto, Nitta and Murata 18 The JECS is an ongoing nationwide birth cohort study that aims to recruit about 100,000 pregnant women and their partners over a 3-year period, collect biological samples and record data on their children from birth to age 13.
In the present study, we examined associations between paternal height or BMI and infant birth weight using the JECS database.
Participants and methods
The JECS is the third largest birth cohort survey following surveys conducted in Denmark and Norway. Details regarding the JECS recruitment and sampling strategy as well as data collection procedures have been described earlier.Reference Kawamoto, Nitta and Murata 18 The pregnant women who were recruited for the JECS study live in one of the 15 study areas throughout the country.
The eligibility criteria for participants (pregnant women) were as follows: (1) residing in the study area at the time of recruitment; (2) expected delivery date after August 1, 2011 and (3) capable of comprehending the Japanese language and completing the self-administered questionnaire. Individuals who resided outside the study area but attended cooperating health care providers within the study area were excluded from the study. Three self-administered questionnaires and four medical record-transfer questionnaires were collected during pregnancy and one month postpartum. Clinical measurements and biological samples, such as those typically obtained during pregnancy, were collected by trained nurses, physicians, midwives or research coordinators.
The JECS was conducted in accordance with the Helsinki Declaration and other national regulations and guidelines, and all participants of the JECS provided informed consent.Reference Kawamoto, Nitta and Murata 18 , 19 The JECS protocol was approved by the Ministry of the Environment epidemiological studies review board and the ethics committees of all participating institutions.
Data collection
The present study used dataset jecs-ag-20160424, which was released in June 2016 and revised in October 2016, along with supplementary dataset jecs-ag-20160424-sp1. Serial measurements of maternal and paternal weight (kg), height (cm) and BMI (kg/m2) were collected. For the current analysis, maternal (pre-pregnancy) and paternal measurements obtained on the date closest to the infant’s birth were selected. We contacted pregnant women via cooperating health care providers and local government offices issuing Maternal and Child Health Handbooks and registered those willing to participate. Whenever possible, the women’s partners (fathers) were also approached and encouraged to participate.
Infant measurements were obtained from the JECS database. Self-administered questionnaires, which were completed by the women during the first trimester (MT1) and second/third trimester, collected information on demographic factors, medical and obstetric history, physical and mental health, lifestyle, occupation, environmental exposures at home and in the workplace, housing conditions and socioeconomic status. Between the mothers’ early pregnancy and 1 month after delivery, their male partners were asked to complete a questionnaire.Reference Michikawa, Nitta and Nakayama 20 Following the standard operating procedures, physicians, midwives, nurses and research coordinators transcribed relevant information from medical records. LGA was defined as a birth weight within or above the 90th percentile of Japanese infants.Reference Itabashi, Fujimura and Kusuda 21 SGA was defined as a birth weight below the 10th percentile of Japanese infants.Reference Itabashi, Fujimura and Kusuda 21 AGA was defined as a birth weight within or above the 10th percentile and below the 90th percentile of Japanese infants.Reference Itabashi, Fujimura and Kusuda 21 Clinically impossible values including maternal height <67.6 or >558 cm, or maternal body weight <7 or >356 kg were treated as missing data.
Statistical analysis
In the analyses of differences in maternal and paternal characteristics according to categories of paternal height, continuous variables were tested with a general linear model. Differences in categorical variables were tested with χ2 tests.
To evaluate the association between paternal height and SGA or LGA, a multinomial logistic regression model was applied. After being divided into quartiles, paternal height was included in the model as a categorical variable. Next, the linear associations between paternal height and SGA or LGA were tested. Finally, paternal height was included in the model as a continuous variable.
All models were adjusted for annual income, conception method, marital status, paternal variables and maternal variables. Paternal variables included in the model were age, BMI, smoking status, alcohol consumption, highest level of education and gestational age at the first trimester questionnaire for fathers (FT1). Maternal variables included in the model were age; height; pre-pregnancy BMI; highest level of education; smoking status at MT1; alcohol consumption at MT1; history of hypertension, diabetes mellitus, kidney disorder or mental illness; folate supplementation at MT1; Kessler 6 (K6) ⩾13 at MT1 and gestational age at MT1.Reference Furukawa, Kawakami, Saitoh, Ono and Nakane 22
Because several variables had missing data, multiple imputation using Markov chain Monte-Carlo simulation was applied.Reference Mark 23 Paternal BMI, categories of infant birth weight (SGA, AGA and LGA), gestational weight gain (GWG), hypertensive disorders of pregnancy and gestational diabetes mellitus were included in the imputation model. After five datasets were created, the same analyses were conducted. Subsequently, the five sets of results were combined and are reported in this manuscript.
Next, the associations between paternal BMI and SGA or LGA were evaluated using a multinomial logistic regression model. Paternal BMI was divided into quartiles and included in the model as a categorical variable. Then, the linear associations between paternal BMI and SGA or LGA were tested. Finally, paternal BMI was included in the model as a continuous variable. All models were adjusted for annual income, conception method, marital status, paternal variables and maternal variables. Paternal variables included in the model were age, height, smoking status, alcohol consumption, highest level of education and gestational age at FT1. Maternal variables included in the model were age; height; pre-pregnancy BMI; highest level of education; smoking status at MT1; alcohol consumption at MT1; history of hypertension, kidney disorder and mental illness; supplementation of folate at MT1; K6 ⩾13 at MT1 and gestational age at MT1. In the same manner as the analysis of paternal height and SGA or LGA, multiple imputation using Markov chain Monte-Carlo simulation was conducted. A two-sided P-value of <0.05 indicated statistical significance. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Parental and neonatal characteristics
Of the 103,099 pregnancies, 69,651 (67.6%) were excluded due to inadequate data, multiple pregnancies, declining consent to participate in this study and other reasons (Fig. 1). First, to examine paternal characteristics, the subjects were divided into the following four groups according to paternal height: Quartile 1 (<168.1 cm), Quartile 2 (168.1–171.9 cm), Quartile 3 (172.0–175.4 cm) and Quartile 4 (⩾175.5 cm). The total number of fathers was 17,078. The number of fathers in Quartiles 1–4 was 4517, 3941, 4336 and 4284, respectively (Table 1). Paternal smoking and drinking rates during pregnancy were 40.6 and 75.1%, respectively. Alcohol consumption and the highest level of education were significantly different across paternal height groups.

Fig. 1 Flow diagram of participants summarizing inadequate records and missing data The study analyzed data of 33,448 out of the 103,099 pregnant women who provided primary fixed data from the Japan Environment and Children’s Study.
Table 1 Paternal characteristics by paternal height of male infants

FT1, questionnaire in the first trimester of pregnancy for fathers; BMI, body mass index.
Data are expressed as mean (s.d.) or percentages, unless otherwise noted.
The subjects were divided into the following four groups according to paternal height (Table 2). As shown in Table 2, the total number of fathers was 16,370 and the number of fathers in Quartiles 1–4 was 4354, 3769, 4142 and 4105, respectively. Paternal smoking and drinking rates during pregnancy were 41.2 and 75.0%, respectively. Alcohol consumption and the highest level of education were significantly different across the paternal height quartiles.
Table 2 Paternal characteristics by paternal height of female infants
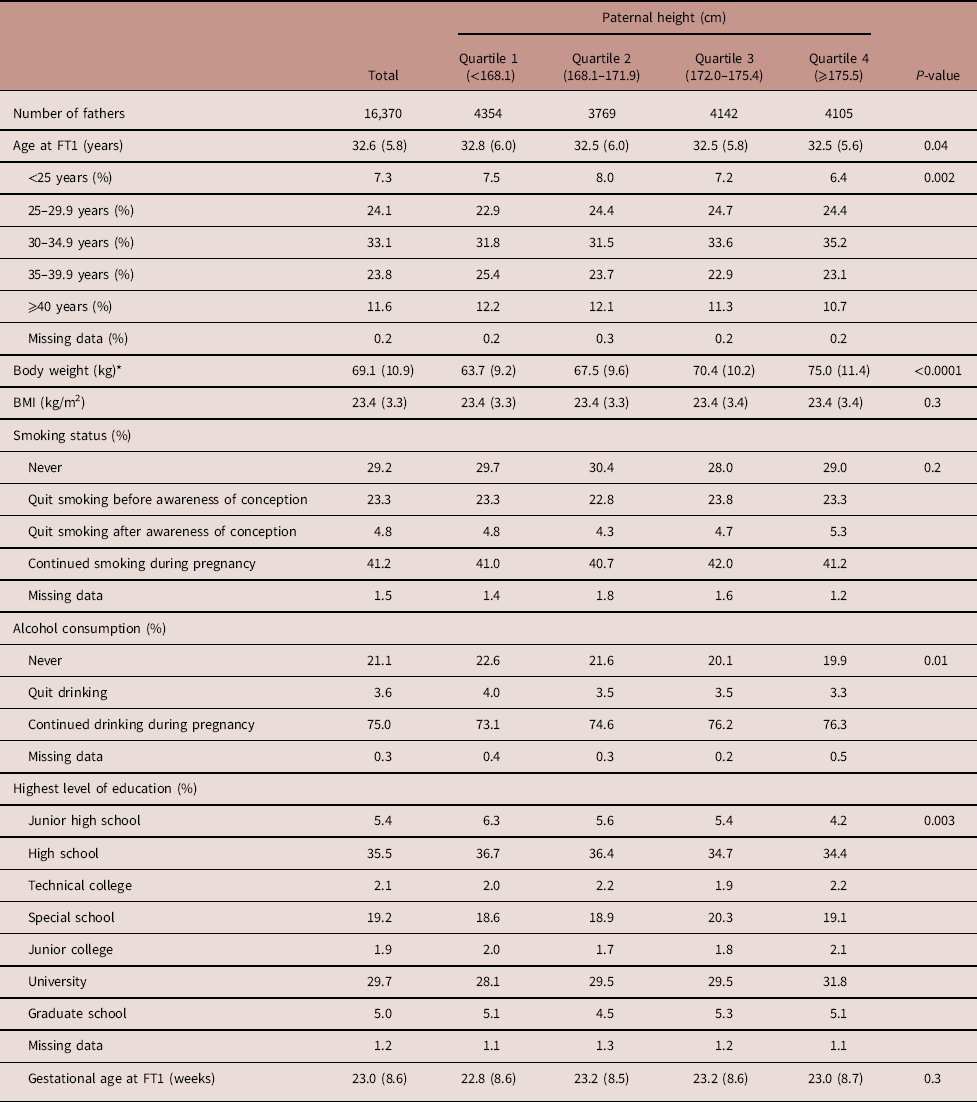
FT1, questionnaire in the first trimester of pregnancy for fathers; BMI, body mass index.
Data are expressed as mean (s.d.) or percentages, unless otherwise noted.
Maternal characteristics of male and female infants along with paternal height categories are shown in Tables 3 and 4, respectively. As shown in Table 3, the primipara rate was 49.5%. Both maternal height and BMI increased as paternal height increased. GWG was different across the paternal height quartiles. The maternal smoking and drinking rates during pregnancy were 4.0 and 9.7%, respectively. Smoking status, the highest level of education and annual income were also significantly different across the paternal height groups. Table 4 shows similar results as Table 3.
Table 3 Maternal characteristics by paternal height of male infants

FT1, questionnaire in the first trimester of pregnancy for fathers; BMI, body mass index, MT1, questionnaire in the first trimester of pregnancy for mothers; GWG, gestational weight gain; ART, assisted reproductive technology.
Data are expressed as mean (s.d.) or percentages, unless otherwise noted.
*The number of missing data points were as follows: maternal age at MT1 (n=1), maternal height (n=126), maternal body weight (n=273), maternal BMI (n=281) and GWG (n=596).
Table 4 Maternal characteristics by paternal height of female infants
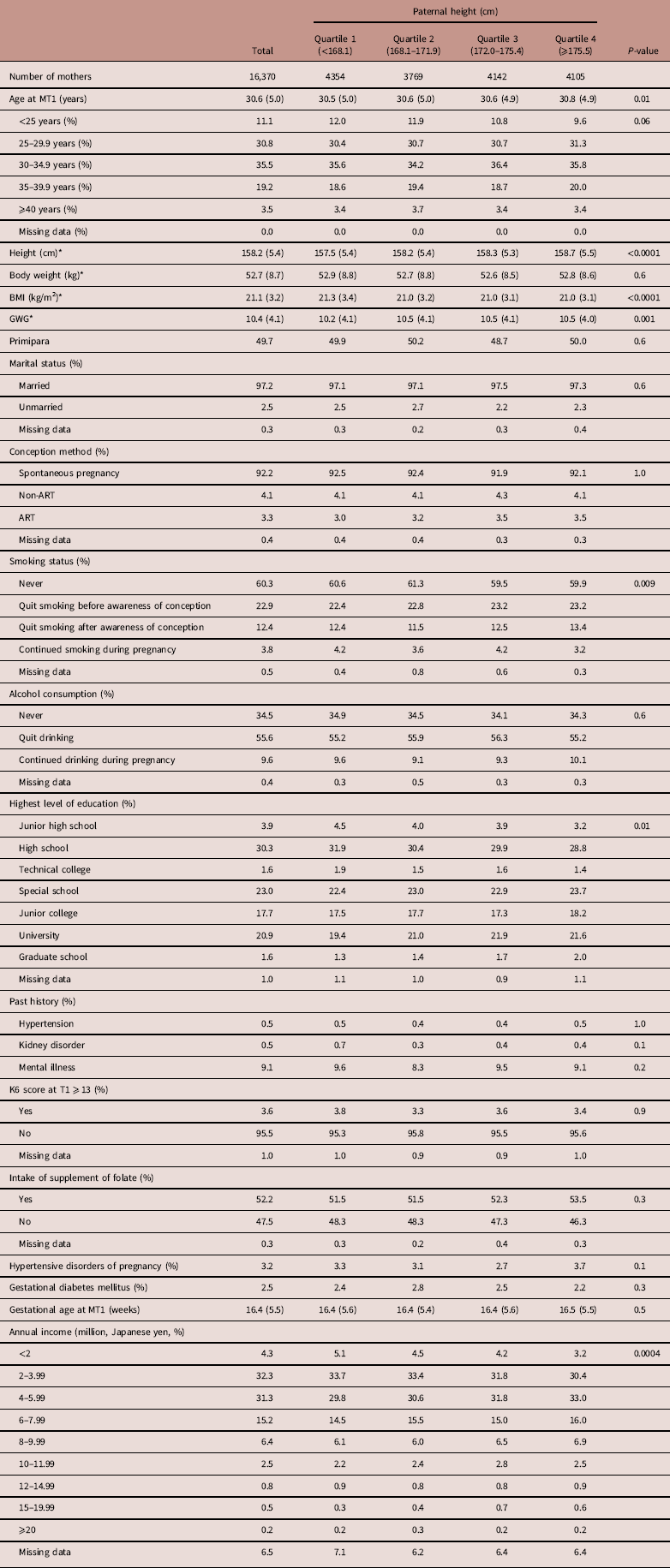
MT1, questionnaire in the first trimester of pregnancy for mothers; BMI, body mass index; GWG, gestational weight gain; ART, assisted reproductive technology; K6, Kessler 6; T1, questionnaire in the first trimester.
Data are expressed as mean (s.d.) or percentages, unless otherwise noted.
*The number of missing data points were as follows: maternal height (n=111), maternal body weight (n=253), maternal BMI (n=259) and GWG (n=562).
Neonatal characteristics of male and female infants are shown in Tables 5 and 6, respectively. Similar results were recognized between neonatal characteristics of male and female infants. Gestational age at delivery and the preterm delivery rate showed no significant difference between male and female infants across paternal height quartiles.
Table 5 Neonatal characteristics by paternal height of male infants
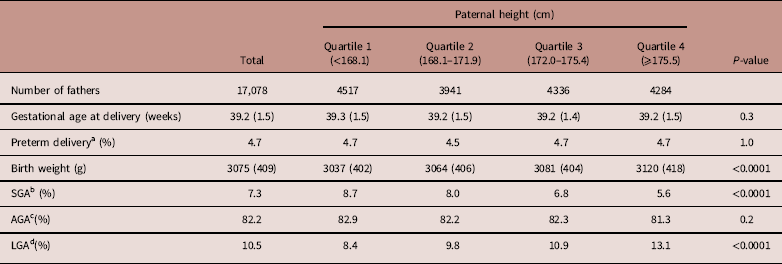
Data are expressed as mean (s.d.) or percentages, unless otherwise noted.
a Preterm delivery was defined as delivery before 37 weeks of gestation.
b SGA was defined as a birth weight below the 10th percentile of Japanese infants.
c AGA was defined as a birth weight within or above the 10th percentile and below the 90th percentile of Japanese infants.
d LGA was defined as a birth weight within or above the 90th percentile of Japanese infants.
Table 6 Neonatal characteristics by paternal height of female infants

SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
Data are expressed as mean (s.d.) or percentages, unless otherwise noted.
a Preterm delivery was defined as delivery before 37 weeks of gestation.
b SGA was defined as a birth weight below the 10th percentile of Japanese infants.
c AGA was defined as a birth weight within or above the 10th percentile and below the 90th percentile of Japanese infants.
d LGA was defined as a birth weight within or above the 90th percentile of Japanese infants.
Association between paternal height and birth weight of their offspring
Multivariate analysis showed that higher paternal height was associated with lower odds of SGA in both male and female infants (Table 7). The significance of the trend in both male and female infants was P<0.0001. Higher paternal height was associated with higher odds of LGA. The significance of this trend in both male and female infants was P<0.0001. Paternal height showed a similar impact on the odds of SGA and LGA in both male and female infants.
Table 7 Association between paternal height and birth weight of infant a

OR, odds ratio; CI, confidence interval; SD, standard deviation; SGA, small for gestational age; LGA, large for gestational age; BMI, body mass index; MT1, questionnaire in the first trimester of pregnancy for mothers.
a All models were adjusted by annual income, conception method, marital status, paternal variables and maternal variables. Paternal variables included in the model were age, BMI, smoking status, alcohol consumption, highest level of education and gestational age at the first trimester questionnaire for fathers. Maternal variables included in the model were age; height; pre-pregnancy BMI; highest level of education; smoking status at MT1; alcohol consumption at MT1; past histories of hypertension, kidney disorder and mental illness; supplementation of folate at MT1; Kessler 6 ⩾13 at MT1 and gestational age at MT1.
b 1 s.d.=5.7 cm for paternal height.
Comparison of the effects of paternal height and paternal BMI on birth weight of their offspring
Comparison of the association between paternal height and paternal BMI with the odds of SGA or LGA in male and female infants is shown in Table 8. In the case of male infants, the effects of paternal BMI on the odds of either SGA or LGA were similar to those of paternal height. However, paternal height had a stronger impact than paternal BMI on the odds of male LGA. Higher paternal height was related to lower odds of SGA in female infants. On the other hand, paternal BMI showed no association with the odds of SGA in female infants. Higher paternal height was related to higher odds of LGA in female infants. Paternal BMI showed only a weak association with the odds of LGA in female infants.
Table 8 Multivariable regression analysis of the association between paternal height or BMI and birth weight of male and female infants a

OR, odds ratio; CI, confidence interval; SD, standard deviation; SGA, small for gestational age; LGA, large for gestational age; BMI, body mass index; MT1, questionnaire in the first trimester of pregnancy for mothers.
a All models were adjusted by annual income, conception method, marital status, paternal variables and maternal variables. Paternal variables included in the model were age, BMI, smoking status, alcohol consumption, highest level of education and gestational age at the first trimester questionnaire for fathers. Maternal variables included in the model were age; height; pre-pregnancy BMI; highest level of education; smoking status at MT1; alcohol consumption at MT1; past histories of hypertension, diabetes mellitus, kidney disorder and mental illness; supplementation of folate at MT1; Kessler 6 ⩾13 at MT1 and gestational age at MT1.
b 1 s.d.=5.7 cm for paternal height and 3.3 kg/m2 for paternal BMI.
Discussion
The present cohort study took advantage of a large Japanese database that enabled us to clarify the association between paternal height or BMI and birth weight of their offspring. We found that paternal height was associated with birth weight of their offspring and had stronger effects on fetal growth than paternal BMI did in a large-scale Japanese population.
These results are consistent with previous studies. A previous study from 1954Reference Cawley, McKeown and Record 24 and two birth cohort studiesReference Cawley, McKeown and Record 24 , Reference Pritchard, Sutherland and Carr-Hill 25 showed a positive relationship between paternal height and birth weight. However, these studies did not use statistical analysis. A meta-analysis reported that increased birth weight was associated with increased paternal height in all 10 studies reviewed.Reference Shar 26 Other recent studies not included in this meta-analysis also showed similar results.Reference Morrison, Williams, Najman and Andersen 11 – Reference Kawamoto, Nitta and Murata 18 More recently, Pomeroy et al. reported paternal body size was particularly associated with limb length.Reference Pomeroy, Wells, Cole, O’Callaghan and Stock 27 Therefore, taking the results of these previous reports and the present study together, the effects of paternal height on fetal growth appear to be of genetic origin.
On the other hand, studies of paternal BMI and its influence on birth weight show contradictory results. Several studies have examined the relationship between paternal BMI and birth weight. Knight et al. reported that paternal BMI has little effect on birth weight,Reference Knight, Shields and Hill 9 and several other studies showed that paternal BMI was not associated with birth weight.Reference Morrison, Williams, Najman and Andersen 11 – Reference Kawamoto, Nitta and Murata 18 The strengths of the present study are the large number of participants and the adjustments for various confounding maternal and paternal factors that may also influence birth weight. Therefore, this study partially supports the previous studies. Consistent with the present study, Pomeroy et al. showed that maternal and paternal height and maternal BMI were associated with birth weight of their infant.Reference Pomeroy, Wells, Cole, O’Callaghan and Stock 27 Furthermore, they reported that paternal body size was associated with trunk and limb lengths, while maternal height and BMI were more strongly associated with adiposity such as neonatal skinfold and birth weight.Reference Pomeroy, Wells, Cole, O’Callaghan and Stock 27 Our results showed that although paternal height was associated with infant birth height, paternal BMI was not associated with infant birth height (data not shown). Based on these observations, neonatal trunk and limb size may be determined genetically by paternal factors and neonatal adiposity may be determined epigenetically by maternal factors.
Interestingly, in our study, paternal BMI affected the prevalence of SGA and LGA in male infants but paternal BMI had a weak effect on the prevalence of SGA and LGA in female infants. These results are similar to a previous study by Chen et al. that showed paternal BMI affects the growth of male but not female infants.Reference Chen, Xiao and Li 28 Earlier studies rarely examined statistical differences between male and female infants in terms of the relationship between birth weight and parental phenotype. Both paternal genetics and epigenetics help to determine birth weight. There are few epidemiological studies to support paternal epigenetic effects. One such study by Pembrey et al. reported that paternal famine caused a higher risk of obesity and cardiovascular disease two generations later.Reference Pembrey, Bygren and Kaati 29 Animal experiments also demonstrated that paternal obesity is associated with the offspring’s phenotype in a sex-dependent manner. For instance, Ng et al. showed that a high-fat diet in fathers programs β-cell dysfunction in female rat offspring at 6 weeks of age is through epigenetic mechanisms.Reference Ng, Lin and Laybutt 30 Furthermore, they reported that paternal high-fat feeding induces common changes in the transcriptomes of adipose and pancreatic islet tissues in female rat offspring.Reference Ng, Lin and Maloney 31 Terashima et al. showed that a high-fat diet in male mice caused alteration of gene expression in the liver by modulation of histone composition.Reference Terashima, Barbour and Ren 32 These reports suggest that epigenetic alterations would occur. However, the underlying mechanism of the alterations is still unclear. Further studies based on the prospective JECS data along with experimental studies using mice model are required to further address this question.
This study had some limitations. First, paternal height and the weights of both parents were self-reported and are therefore subject to bias.Reference Gorber, Tremblay, Moher and Gorber 33 However, BMI based on self-reported measurements may be sufficiently accurate for epidemiological studies.Reference McAdams, Van Dam and Hu 34 Second, 67.6% of all the subjects were excluded mainly due to declining consent to participate in this study. Therefore, selection bias is present. However, external validity will not be low because maternal age, birth weight and gestational age were similar with Japanese general population. Third, we do not present detailed results of differences in the impact of maternal or paternal BMI on birth weight because these results will be presented in a future study. As shown in Supplementary Tables S1 and S2, our study demonstrated that although maternal and paternal height showed similar effects on birth weight, the impact of maternal BMI on birth weight was stronger than that of paternal BMI. These findings agree with previous studies. Pomeroy et al.Reference Pomeroy, Wells, Cole, O’Callaghan and Stock 27 and Lawlor et al.Reference Lawlor, Smith and O’Callaghan 35 demonstrated that the impact of maternal BMI on birth weight is stronger than that of paternal BMI. Some investigators have examined the associations between both maternal and postnatal early childhood BMI and paternal and postnatal early childhood BMI. However, they found inconsistent results and had important limitations.Reference Sekine, Yamagami and Hamanishi 36 , Reference Esposito-Del, Scalfi and De 37 Also, placental weight is associated with birth weight; therefore the effects of paternal height or body weight on placental weight will be examined in a future project. Many studies have reported correlations between maternal BMI and birth weight, BMI and adiposity from birth to 3 years,Reference McIntire, Bloom, Casey and Leveno 2 , Reference Mei, Grummer-Strawn, Thompson and Dietz 3 although some studies have not found this association.Reference Koyanagi, Zhang and Dagvadorj 1 , Reference Barker, Hales and Fall 4 , Reference Wei, Sung and Li 5 Recently, Morgan et al. showed that parental BMI, low socioeconomic status and smoking during pregnancy have persistent, strong and direct effects on child BMI and overweight status at age 7, independent of birth weight and infancy BMI. 38 Moreover, Mikkelsen et al. reported that paternal obesity is associated with a higher risk of behavioral problems in children.Reference Mikkelsen, Hohwü and Olsen 39 Furthermore, the Northern Finland cohort showed that paternal overweight conveys a risk of overweight in children after a 16-year follow-up.Reference Pirkola, Pouta and Bloigu 40 Therefore, further examination of the relationship between paternal BMI and NCDs of offspring later in life is necessary. Accumulation of such studies could provide the cornerstone of NCD prevention.
In conclusion, we demonstrated that the impact of both paternal height and BMI on birth weight, adjusted for many confounding factors, was correlated with fetal growth. The association between paternal height and birth weight along with maternal height and BMI may affect childhood growth through both the uterine environment and paternal genetic effects.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174418001162
Acknowledgments
The authors would like to express our gratitude to all of the JECS study participants and to the co-operating health care providers. They acknowledge the contributions of the following members of the Japan Environment and Children’s Study (JECS) as of 2017 (principal investigator, Toshihiro Kawamoto): Hirohisa Saito (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Michihiro Kamijima (Nagoya City University, Nagoya, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Yasuaki Hirooka (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Financial support
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation in Japan and with the Helsinki Declaration of 1975, as revised in 2008. The JECS protocol was approved by the Ministry of the Environment epidemiological studies review board and the ethics committees of all participating institutions. All participants of the JECS study provided informed consent.











