Introduction
In vitro fertilization and embryo transfer (IVF-ET) has been increasingly used since it was first reported in 1978, and almost 8 million babies had been born after IVF-ET worldwide every year.Reference Adamson, de Mouzon and Chambers1 However, studies revealed higher risks of low birthweight (LBW) and small for gestational age (SGA), preterm delivery, intrauterine growth restriction, placental abnormality, gestational diabetes, and gestational hypertension in IVF-ET compared with spontaneous conception.Reference Qin, Liu and Sheng2–Reference Royster, Krishnamoorthy and Csokmay5 In this circumstance, awareness and concerns related to the adverse perinatal outcomes associated with IVF-ET have become increasingly prominent.
Controlled ovarian stimulation (COH) has taken an important role in the procedure of IVF-ET to obtain a higher number of oocytes.Reference Pacchiarotti, Selman and Valeri6 Notably, maternal serum estradiol (E2) levels induced by COH were 10–20 times higher than normal.Reference Kalra, Ratcliffe, Coutifaris, Molinaro and Barnhart7 Although higher E2 level has been regarded as indicating a better response to ovarian stimulation, exposure to supraphysiological E2 during pregnancy might lead to negative perinatal and neonatal outcomes. According to several studies, high E2 level induced by COH might sustain for more than 8 weeks following fresh embryo transfer, and lead to adverse effects on endometrial receptivity and intrauterine fetal growth.Reference Hu, Feng and Lin8,Reference Kyrou, Popovic-Todorovic and Fatemi9 Despite the fact that E2 in ovarian follicular fluid from regularly menstrauting women was found up to 200- to 1000-fold higher than in serum,Reference Kushnir, Naessen and Kirilovas10 few studies focus on the impact of oocyte exposure to supraphysiological E2 during COH on further pregnancy outcomes.
Frozen-thawed embryo transfer (FET) enables women who have undergone COH to recover their initial hormone levels before embryo transfer. Although FET appears to partially protect from the adverse effects of high maternal E2 during early pregnancy, a 10-year national cohort from the Nordic countries indicated that the risk of LBW or SGA after FET was still higher than that after spontaneous conception.Reference Wennerholm, Henningsen and Romundstad11,Reference Pelkonen, Koivunen and Gissler12 Thus, it is of great interest to investigate whether high E2 level induced by COH affects the oocyte maturation and lead to adverse perinatal outcomes following FET.
With a prominent lower level of maternal E2 during implantation and early pregnancy, FET cycles become an ideal model to investigate the potential associations between oocyte exposure to markedly increased E2 levels during COH and both short- and long-term outcomes including pregnancy rates, adverse obstetric complications, and perinatal outcomes.
Methods
Study design and participants
This retrospective cohort study included all FET cycles from January 2014 to September 2017 at International Peace Maternity and Child Health Hospital (IPMCHH). Women of advanced age (over 40 years) undergoing FET were excluded due to their increased probability of abnormal basal hormone levels with reduced ovarian reserve. Women who received donated oocytes or sperm or those who underwent preimplantation genetic testing were excluded. Mixed transfers with two embryos retrieved from different oocyte retrieval cycles were also excluded. All cycles were then categorized into three groups according to the maternal serum E2 level on the day of the human chorionic gonadotropin (hCG) trigger (Group I: <10,000 pmol/l; Group II: 10,000–15,000 pmol/l; Group III: >15,000 pmol/l). The cutoff value of 10,000 pmol/l was selected based on our previous observation that E2 values above this level had adverse effects on the offspring.Reference Hu, Feng and Lin8 The cutoff value of 15,000 pmol/l was selected based on the documented increased risk of a subsequent OHSS among patients with an E2 level over 15,000 pmol/l.Reference Mocanu, Redmond and Hennelly13,Reference Morris, Paulson and Sauer14
Ethical approval for the study was obtained from the Institutional Review Board of IPMCHH (GKLW-2016-21). Written informed consent was obtained from all participants before inclusion.
The datasets used and/or analyzed during the current study are available from ResMan Research Manager of Chinese Clinical Trial Registry with the agreement of corresponding authors on reasonable request.
ART Procedures
The process of IVF was conducted according to our standard protocols, including ovarian stimulation, oocyte retrieval, and insemination by either conventional IVF or intracytoplasmic sperm injection. FET was performed following endometrial preparation by natural monitoring, an ovarian stimulation cycle, or hormone replacement therapy. Serum hormone levels, including E2, progesterone (P4), and luteinizing hormone (LH) were detected in the hospital clinical chemistry laboratory on the day of hCG trigger and before embryo transfer separately. Endometrial thickness before FET was measured by highly trained sonographers via transvaginal ultrasound.
Patient data regarding the ART procedure including oocyte retrieval and embryo transfer were collected from the patient’s hospital records. Information that was documented included the type of COH protocol (gonadotropin-releasing hormone [GnRH]-agonist protocol, GnRH-antagonist protocol, the microflare protocol, natural cycles or others), the type of insemination (IVF or intracytoplasmic sperm injection), number of oocytes retrieved (≤10, 11–20 or >20), duration of embryo cryopreservation (<3, 3–6, or >6 months), the type of endometrium preparation (natural cycle, hormone replace therapy cycle, or ovarian stimulation cycle), the day of embryo transfer (day 2, day 3, or day 5), and the number of embryos transferred (1 or 2). Pregnancy outcomes and relevant obstetric outcomes were followed up as previously described.Reference Wu, Li and Zhu15
Outcomes measurements and variable specification
Participants were interviewed in person to obtain information on sociodemographic characteristics and reproductive history. The height and weight were measured, and body mass index (BMI) was calculated before interview. Queries to which the participants did not reply were considered as missing data.
Pregnancy outcome measures following FET were assessed by the serum β-hCG level and ultrasound scans. Additionally, adverse outcomes including ectopic pregnancy, early miscarriage before 12 gestational weeks, stillbirth, and pregnancy termination due to fetal defects were also assessed.
Obstetric complications and neonatal outcomes were abstracted from the participants’ health records, including gestational hypertensive disorder (gestational hypertension, mild preeclampsia, or wild preeclampsia), gestational diabetes mellitus, intrahepatic cholestasis of pregnancy, meconium staining of the amniotic fluid, vanishing twin, mode of delivery (vaginal or caesarean section), birthweight, and gender of neonates. Large for gestational age (LGA), SGA, and appropriate for gestational age were defined according to a global reference for fetal weight and birthweight of our population for a given gestational age and sex.Reference Mikolajczyk, Zhang and Betran16
Statistical analysis
Continuous variables with a normal distribution are represented as the means ± standard deviations, and differences among groups were tested by one-way analysis of variance. Continuous variables with a skewed distribution are represented as medians and interquartile ranges, and differences were tested by the Kruskal–Wallis test. Categorical outcome variables are represented as frequencies with proportions, and differences between each group were modeled using multinomial logistic regression, differences for trend were detected by the Cochran–Mantel–Haenszel χ 2 test.
Obstetric and neonatal outcomes were stratified according to singleton or multiple deliveries. The odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated using logistic regression, to estimate the relationships between supraphysiological E2 exposure during COH and each outcome following FET. To analyze the obstetric complications and neonatal outcomes of singletons, multinomial logistic regression analyses were also performed to adjust ORs for potential confounding factors. When analyzing the neonatal outcomes of multiples, ORs and 95%CIs were obtained using multilevel logistic regression and adjusted for the corresponding confounding factors, according to Carlin et al.Reference Carlin, Gurrin and Sterne17
SAS software version 9.3 (SAS Institute, Inc, Cary, NC) was used to perform all statistical analyses. All p values were calculated using two-sided tests. Differences between values were considered statistically significant at a p value of less than 0.05. For multiple comparison, p values of less than 0.017 were considered to be statistically different.
Results
The flow of the study participants was shown in Fig. 1. A total of 10,581 FET cycles met the eligibility criteria and were included in the analysis (3695 cycles in Group I, 2565 cycles in Group II, and 4321 cycles in Group III). Women with live-born babies (2877 live births with 2230 singletons and 1295 multiples) were included in the analysis of obstetric complications and neonatal outcomes, with 123 participants lost to follow-up.

Fig. 1. Study flow chart. (a) PGT, preimplantation genetic testing. (b) Mixed transfer cycle was defined as transferring two embryos from different oocyte retrieval cycles. (c) Early miscarriage was defined as spontaneous loss of pregnancy before 12 gestational weeks. (d) Late miscarriage was defined as pregnancy loss between 12 and 28 gestational weeks.
Reproduction history including maternal age at oocyte retrieval and at embryo transfer, pregestational BMI, duration of infertility, primary infertility, and causes of infertility were similar among three groups (see Table 1). However, the distribution of COH protocols administered was different among groups (p < 0.001). Patients with higher E2 level on the day of hCG trigger were more likely to have experienced GnRH-agonist protocol, while those with lower E2 level were more likely to have experienced microflare protocol. Furthermore, the number of oocytes retrieved tended to increase as E2 levels increased on the day of hCG trigger (p < 0.001). Notably, serum levels of P4 and LH showed no differences on the day of hCG trigger among groups, and no differences were found in endometrial thickness and hormone levels before FET among three groups either.
Table 1. Baseline characteristics of all FET cycles
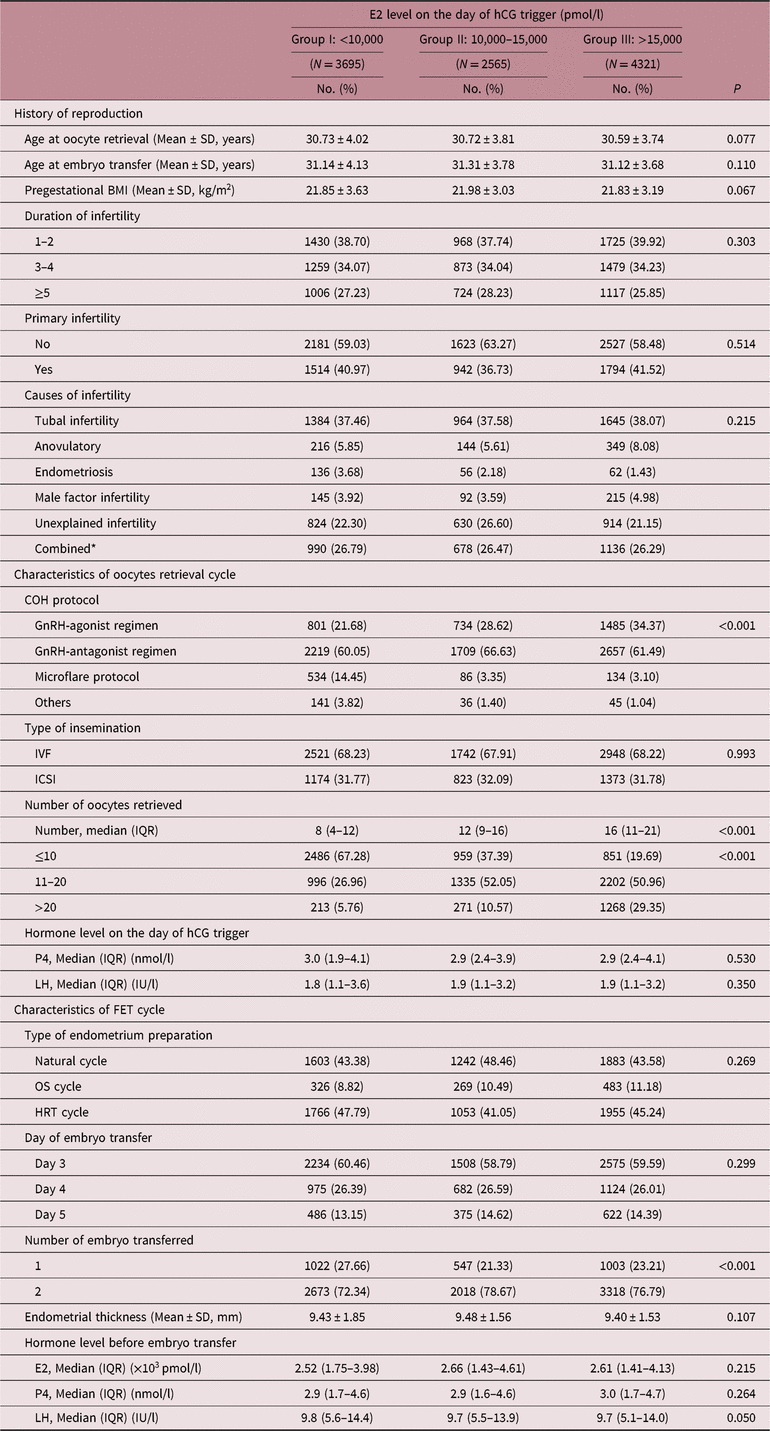
FET, frozen embryo transfer; BMI, body mass index; COH, controlled ovarian stimulation; ART, assisted reproductive technology; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; OS, ovarian stimulation; HRT, hormonal replace therapy; E2, estradiol; P4, progesterone; LH, luteinizing hormone.
* Combined was defined as two or more infertile causes mentioned above.
Table 2 shows the pregnancy outcomes per transfer cycle of the three groups. After confounding factors were adjusted, patients with higher E2 level on the day of hCG trigger showed lower percentage of chemical pregnancies (Group I: 43.17%, Group II: 39.22%, Group III: 38.97%, p trend < 0.001), clinical pregnancies (Group I: 36.75%, Group II: 34.46%, Group III: 33.93%, p trend = 0.009), ongoing pregnancies (Group I: 31.50%, Group II: 28.62%, Group III: 28.00%, p trend < 0.001), and live births (Group I: 28.80%, Group II: 26.90%, Group III: 25.99%, p trend = 0.005) per transfer cycle than those with lower E2 levels during COH. In addition, the frequency of early pregnancy loss increased as E2 levels increased on the day of hCG trigger (Group I: 14.29%, Group II: 16.86%, Group III: 17.46%, p trend = 0.023). No differences were found in ectopic pregnancy rate (p trend = 0.374), still birth rate (p trend = 0.190), or pregnancy termination rate due to fetal defect (p trend = 0.225). Notably, the fertilization rate decreased as the number of oocytes retrieved increased (Group I: from 83.75% to 70.69%, Group II: from 78.67% to 70.07%, Group III: from 75.62% to 68.93%; Figure S1A). When retrieving less than 10 oocytes, the fertilization rate in Group I was significantly higher than other groups. However, the embryo cleavage rate and the transferrable embryo rate showed no association with the number of oocytes retrieved (Figure S1B–C).
Table 2. Pregnancy outcomes following transferring frozen-thawed embryos with different E2 exposure

a P-values were calculated using multinomial logistic regression, and adjusted for age at oocyte retrieval, pregestational BMI, COH protocol, number of previous ART, number of oocytes retrieved, and number of embryo transferred. P1 for comparisons between Group II and Group I, and p2 for comparisons between Group III and Group I.
b P for trend was calculated using Cochran–Mantel–Haenszel test, and adjusted for age at oocyte retrieval, pregestational BMI, COH protocol, number of oocytes retrieved, and number of embryo transferred.
c Chemical pregnancy was defined as an elevated serum β-hCG level of more than 10 mIU/ml. Chemical pregnancy rate was defined as the number of chemical pregnancy divided by the number of total transfer cycles for each group.
d Clinical pregnancy was defined as a pregnancy documented by ultrasound at 6–8 gestational weeks that shows a gestational sac in the uterus. Clinical pregnancy rate was defined as the number of clinical pregnancy divided by the number of total transfer cycles for each group.
e Ongoing pregnancy was defined as a pregnancy documented by ultrasound at 12 gestational weeks that shows the presence of fetal heartbeat. Ongoing pregnancy was defined as the number of ongoing pregnancy divided by the number of total transfer cycles for each group.
f Live birth was defined as the delivery of one or more infants with any signs of life after 28 weeks of gestation. Live birth rate (% per transferred cycle) was defined as the number of live birth divided by the number of total transfer cycles for each group.
Maternal demographic characteristics and reproductive history of women who delivered live babies were similar among three groups (see Table 3), while distribution of COH protocols and number of oocytes retrieved showed different in both singleton and multiple deliveries (p < 0.001; Table S1). When it comes to FET procedures, no difference was found among three groups (Table S2).
Table 3. Maternal characteristics of pregnancies carried to term following transferring embryos with different E2 exposure
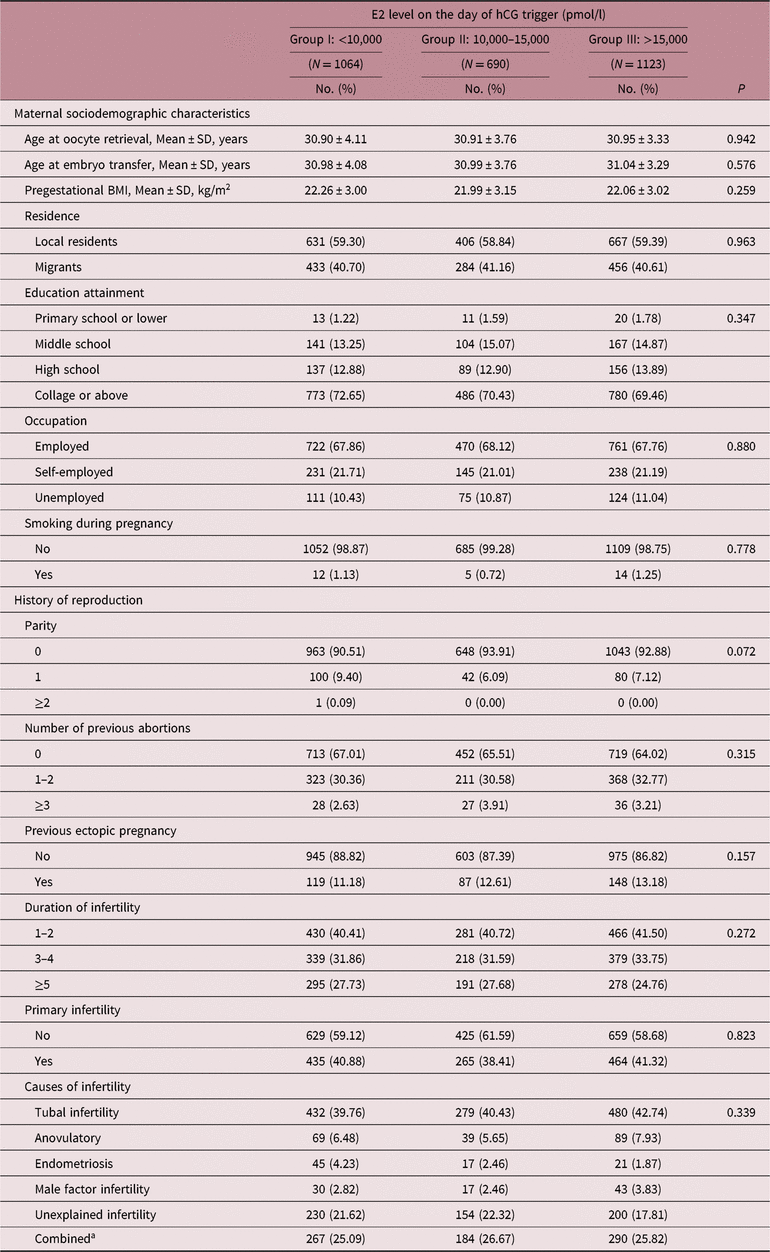
BMI, body mass index.
a Combined was defined as two or more infertile causes mentioned above.
A multivariable analysis of pregnancy complications is shown in Table 4. After adjusting for confounding factors, higher levels of E2 on the day of hCG trigger were found to be associated with an increased risk of preterm birth in singleton deliveries (aOR1 = 1.93, 95% CI: 1.22–3.06; aOR2 = 2.05, 95% CI: 1.33–3.16). Additionally, there were comparable proportions of cases with gestational diabetes mellitus, hypertensive disorder, intrahepatic cholestasis of pregnancy, meconium staining of the amniotic fluid, and caesarean deliveries among the three groups in both singleton and multiple deliveries.
Table 4. Pregnancy complications of pregnancies carried to term following transferring embryos with different E2 exposure
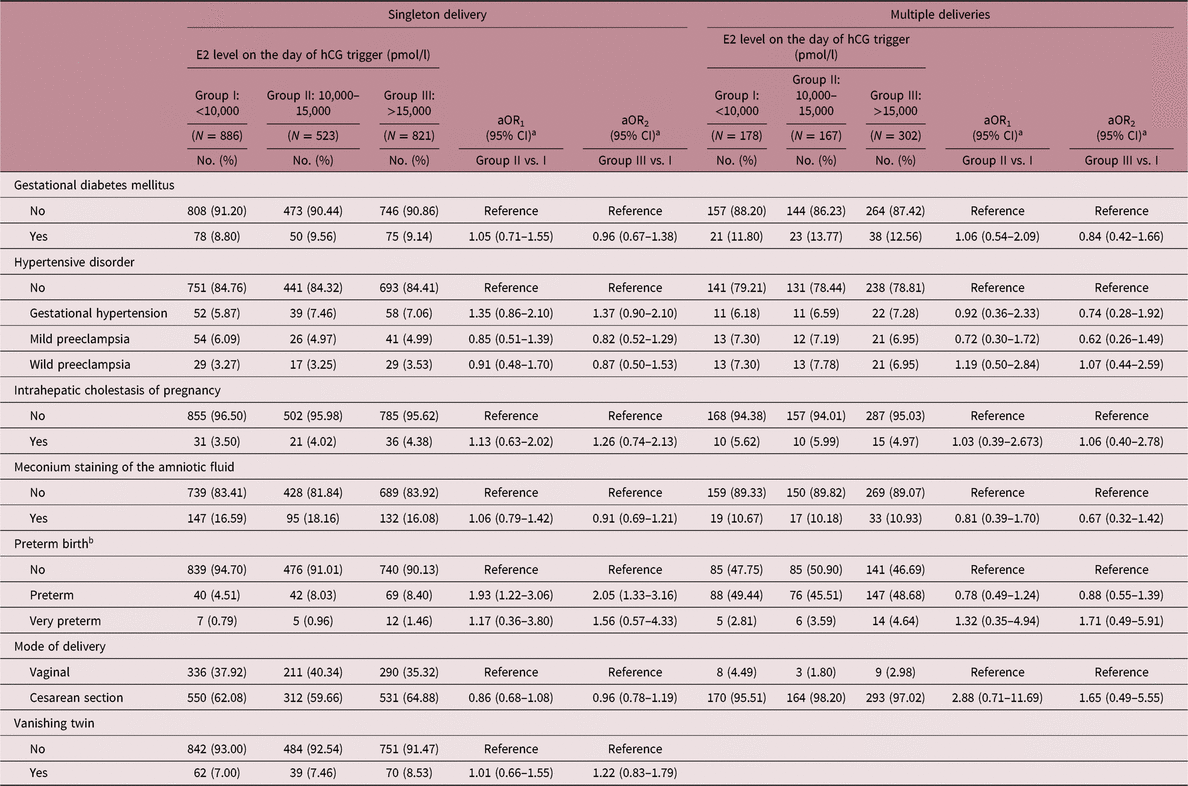
aOR, adjusted odds ratio; CI, confidence interval.
a aOR was adjusted for age at oocyte retrieval, age at embryo transfer, pregestational BMI, COH protocol, number of oocytes retrieved.
b Preterm was defined as delivery of baby before 37 gestational weeks of pregnancy, and very preterm was defined as delivery of baby between 28 and 32 gestational weeks of pregnancy.
Table 5 reports the associations between neonatal outcomes and E2 level on the day of hCG trigger. As the level of E2 increased on the day of hCG trigger, the risk of LBW births following FET also significantly increased in both singleton and multiple deliveries (singleton: aOR1 = 1.27, 95% CI: 0.75–2.26; aOR2 = 1.94, 95% CI: 1.18–3.20; multiples: aOR1 = 1.51, 95% CI: 1.01–2.26; aOR2 = 1.86, 95% CI: 1.02–3.40). Additionally, increased risks of SGA were found in groups with higher levels of E2 in singletons (aOR1 = 2.01, 95% CI: 1.30–3.11; aOR2 = 2.51, 95% CI: 1.69–3.74). A similar effect was also observed in multiple deliveries (aOR1 = 1.58, 95% CI: 1.03–2.45; aOR2 = 1.99, 95% CI: 1.05–3.84). Due to the impact of COH on maternal E2 levels, a subgroup analysis based on COH protocol was also carried out (Table S3). Women experienced either GnRH-agonist regimen or GnRH-antagonist regimen showed higher risks of SGA as E2 level on the day of hCG trigger increased in singletons (GnRH-agonist regimen: aOR2 = 2.16, 95% CI: 1.13–4.11; GnRH-antagonist regimen: aOR2 = 2.40, 95% CI: 1.40–4.11). There was no evidence of differences among the study groups in terms of macrosomia in singletons, and no cases of macrosomia was found in the three groups among multiples. No association was observed between LGA and E2 level on the day of hCG trigger.
Table 5. Outcomes of neonates born following transferring embryos with different E2 exposure
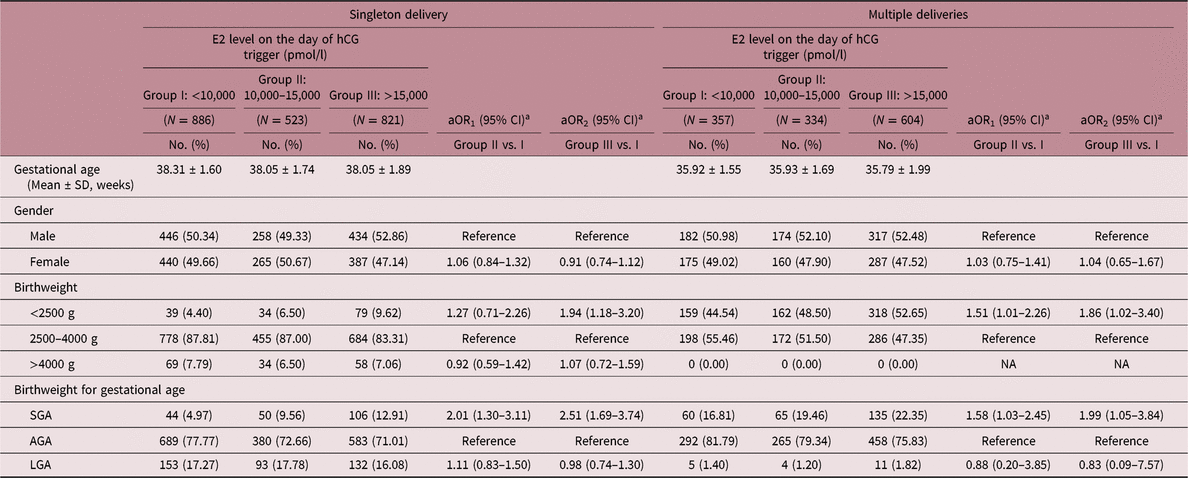
aOR, adjusted odds ratio; CI, confidence interval; NA, not accessible; AGA, appropriate for gestational age; SGA, small for gestational age.
a aOR was adjusted for age at embryo transfer, pregestational BMI, COH protocol, number of oocytes retrieved, number of embryo.
Discussion
In this retrospective cohort of 10,581 FET cycles, we found that high E2 levels on the day of hCG trigger was associated with lower percentage of clinical pregnancy, ongoing pregnancy, and live birth, as well as an increased risk of early pregnancy loss after FET. In addition, newborns after FET with higher E2 levels during COH showed an increased risk of preterm birth in singletons, along with an increased risk of LBW and SGA in both singletons and multiples. These findings strongly suggest that COH-induced high E2 may have adverse effects on oocyte development and maturation, and subsequently increase the risk of SGA following FET.
The most critical discrepancy between fresh embryo transfer and FET is the COH-induced persistent changes in the hormonal milieu after embryo transfer, especially supraphysiological E2 levels.Reference Jarvela, Pelkonen and Uimari18 FET is believed to enable patients who have experienced COH to recover to their initial hormone levels in the next or further cycles, thus providing a relatively normal hormonal milieu during pregnancy.Reference Hu, Feng and Lin8 However, in cases of women who were transferred fresh embryo in the current cycle soon after COH, incidence of SGA was reported to be higher than FET.Reference Pelkonen, Koivunen and Gissler12,Reference Wikland, Hardarson and Hillensjo19,Reference Pinborg, Loft and Aaris20 Moreover, large cohort studies confirmed that the alternation of hormone milieu by COH is one of the interpretations of risk of LBW or SGA in fresh embryo transfer.Reference Kalra, Ratcliffe and Coutifaris4,Reference Pereira, Elias and Christos21,Reference Henningsen, Pinborg and Lidegaard22 Notably, a similar association between COH and LBW or SGA was also found in FET population in this study, regardless of the fact that FET cycles provide a significantly lower level in E2 before embryo transfer than that during COH (E2: 2578 [1518–4171] pmol/l vs. 12796 [7641–17800] pmol/l, p < 0.001, data not shown in tables). Thus, supraphysiological E2 level induced by COH is believed to have influence not only on embryos during early pregnancy, but also on the oocytes during COH. Furthermore, it is worth noting that the rate of fertilization was negatively correlated with the number of oocyte retrieval. A higher number of oocytes retrieved is regarded as an indication of excessive E2 levels during COH,Reference Baker, Brown and Luke23,Reference Sunkara, La Marca and Seed24 as estrogen stimulates follicle development and is synthetized and secreted locally to a higher level by granulosa cells.Reference Kushnir, Naessen and Kirilovas10 According to our findings, in spite of the fact that a certain level of estrogen is essential for the growth of follicles, milder ovarian stimulation regimens with less than 10 oocytes retrieved should be advocated to avoid the excessive response of ovary and impairment on oocyte maturation following IVF.
The process of oocyte maturation is complicated, which involves phenotypic changes, reorganization of cytoplasmic structures, organelle formation, and changes in specific molecules related to fertilization and embryo development. Evidence from animal models and human has indicated that certain transient environmental influences could produce persistent changes in epigenetic marks, thus regulating fetal growth and development in the future life.Reference Fortier, McGraw and Lopes25–Reference Ollikainen, Smith and Joo27 Early in the 2001, Van der Auwera et al. transferred blastocysts from stimulated female mice and naturally cycling controls to non-stimulated foster mothers separately and found that superovulation caused delayed embryonic development and a pronounced fetal growth restriction.Reference Van der Auwera and D’Hooghe28 Furthermore, experiments from nonhuman primates suggest an activated E2 signaling involved in the regulation of trophoblast differentiation in early embryo development,Reference Bonagura, Babischkin and Aberdeen29,Reference Maliqueo, Echiburu and Crisosto30 subsequently leading to insufficient invasion of trophoblast and uteroplacental vessel remodeling. As a result, the blood flow is impaired to the placenta, which might cause several adverse obstetric outcomes, for example, miscarriage, preterm birth, preeclampsia, and SGA.Reference Bonagura, Pepe and Enders31 Although no evidence of an effect was found in regard to gestational hypertensive disorders, an increased risk of preterm birth and SGA after deliveries was confirmed in this study.
Recent years, a growing number of studies indicated that the cause of many chronic diseases in adulthood including obesity, diabetes, hypertension, and cardiovascular diseases can be traced back to gametic and embryonic development stage, and exploring origin of the disease contributes to early intervention and treatment.Reference Barker32–Reference Cooke, Shah and Kirschenman34 However, due to the ethical concerns, interventional study is not permitted on human gametes and embryos. In this study, FET provides us an ideal model to explore the association between the follicle microenvironment and oocyte development as well as further pregnancy outcomes. As this is a hospital-based retrospective cohort study, several confounders related to the medical procedures have been taken into consideration. It is, therefore, not possible to rule out unknown confounders which might influence the risk of obstetric complications and newborn birthweight, such as nutrient intake and physical activities during pregnancy. Despite the limitations, our study is the first epidemiological investigation with regard to the impact of oocyte exposure to transient increase in E2 during COH on pregnancy outcomes, as well as the subsequent perinatal outcomes, without the interference of abnormal maternal hormone milieu during implantation and early pregnancy. The large sample size provided sufficient power to detect small but clinically significant effects. Evidence provided by this study regarding the potential adverse effects of supraphysiological E2 should be taken into consideration when formulating treatment options.
In summary, an increased level of E2 during COH is associated with a decreased pregnancy rate, as well as an increased frequency of early miscarriage following FET. Furthermore, pregnancies conceived from frozen-thawed embryos with supraphysiological E2 exposure during COH may also at increased risk of preterm birth, LBW, and SGA. Therefore, milder COH protocols should be used to avoid the exposure of a supraphysiological level of E2 to the gametes and embryos.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S2040174419000679
Acknowledgements
We thank Nigel Rollins, Maternal, Newborn, Child and Adolescent Health, World Health Organization, for contributions of critical revision of the manuscript. We also thank Prof. Shu-Min Duan, Zhejiang University School of Medicine, for the contributions of study designing.
Financial support
This study was supported by the National Key Research and Development Program of China (2018YFC1002804, 2016YFC1000203), the National Natural Science Foundation of China (81671412, 81661128010), Foundation of Shanghai Municipal Commission of Health and Family Planning (20144Y0110), and Clinical Skills Improvement Foundation of Shanghai Jiaotong University School of Medicine (JQ201717).
Conflict of interest
None.
Ethical standards
Ethical approval for the study was obtained from the Institutional Review Board of IPMCHH (GKLW-2016-21). Written informed consent was obtained from all participants before inclusion.
Author Contributions
He-Feng Huang conceived the hypothesis. Yan-Ting Wu designed the study. Chen-Chi Duan and Cheng Li drafted the manuscript. Yi-Chen He, Jing-Jing Xu, and Chao-Yi Shi conducted the statistical analysis and participated in the discussion. Hong-Tao Hu, Yun-Fei Su, and Lei Chen participated in data collection. Ya-Jing Tan conducted embryo manipulation. Zhi-Wei Liu, Jian-Zhong Sheng, William Fraser, and He-Feng Huang critically revised the manuscript for important intellectual content. All authors commented on the drafts and approved the final draft.








