Introduction
Temperament refers to individual differences in emotional, motor and attentional reactivity measured by latency, intensity and recovery of response, and self-regulation processes such as effortful control. Rothbart and DerryberryReference Rothbart and Derryberry1 suggested that infant temperament can be assessed by the degree to which infants react and regulate their responses. Since reactivity is the response to external stimuli and regulation is the manner in which the infant returns to homeostasis, differences in individual infant reactions can be assessed behaviorally and understood biologically.
An increasing body of literature has suggested a link between temperamental aspects and child psychopathology.Reference Eisenberg, Sadovsky and Spinrad2, Reference Kagan and Snidman3 Specifically, three dimensions of the broad temperamental components have been linked with the development of psychopathology later in lifeReference Nigg, Goldsmith and Sachek4–Reference Rettew, Copeland, Stanger and Hudziak6: activity level (gross motor activity, including squirming and locomotor activity), distress to limitations (fussing, crying or showing distress when confined in a place/position or when unable to perform a desired action) and duration of orienting (attention to and/or interaction with an object). Infants high in distress are at greater risk of being more aggressive.Reference Eisenberg, Cumberland and Spinrad7 A meta-analysis on the relationship between problems in infancy and long-term behavioral outcomes suggests that high distress, usually manifested as excessive crying, in the first year of life is predictive of attention problems at school age.Reference Hemmi, Wolke and Schneider8 Persistent problems with behavioral control, such as the inability to regulate feeding and sleeping behavior in infancy, are precursors of behavior control difficulties such as hyperactivity or conduct problems in childhood.Reference Degangi, Dipietro, Greenspan and Porges9–Reference Wolke, Rizzo and Woods11 Finally, difficulties with anger regulation are associated with attention problems from preschool through adulthood.Reference Douglas and Parry12, Reference Ramirez, Rosén and Deffenbacher13
Previous research has examined the genetic and environmental etiological factors of infant temperament development.Reference Emde and Hewitt 14 – Reference Cherny, Fulker, Corley, Plomin and DeFries 16 Twin studies provide strong evidence of genetic influence on temperament including activity,Reference Wood, Saudino, Rogers, Asherson and Kuntsi 17 distressReference Cherny, Fulker, Corley, Plomin and DeFries 16 and attention.Reference Cyphers, Phillips, Fulker and Mrazek 18, Reference Goldsmith, Buss and Lemery 19 Estimates of heritability suggest that genetic factors account for 20–60% of the variability of temperament within a population with no consistent pattern of differential heritability across dimensions.Reference Saudino 20
The influence of prenatal environment on children’s temperament and behavior is probably less strong than the influence genetic factors have.Reference Knopik, Neiderhiser, de Geus and Boomsma 21 In general, a genetically informative study design helps controlling for a large number of confounders that might affect the results. However, the prenatal environment experienced by twins may be different from the one singletons experience. This is mainly due to space and nutrients scarcity, which could lead to various growth complications.Reference Long and Ferriman 22 Moreover, it is possible that monozygotic (MZ) twins, as compared to singletons and dizygotic (DZ) twins, face a more challenging environment while in the uterus, as a very large proportion of them share one chorion.Reference Marceau, McMaster and Smith 23 However, no study has reported a significant influence of chorionicity on temperament,Reference Marceau, McMaster and Smith 23 while an older studyReference Riese 24 showed that there is no clear pattern of genetic influences in the neonatal period and no significant differences between the MZ and DZ twins correlations, suggesting the presence of shared prenatal environmental influence on temperament.
Since both pregnancy and obesity are associated with massive metabolic alterations acting together, they might impact the environment experienced by the fetus. It has been hypothesized that maternal adiposity at the time of conception may be important for child mental health programming, since the prenatal development of the brain might depend on the level of maternal energy supply.Reference Luke, Dickinson and Petrie 25 , Reference Salvati, Attorri, Avellino, Di Biase and Sanchez 26 However, the possible influence of maternal weight before pregnancy, as a proxy for the prenatal environment, on children psychological development has only recently been studied. Van Lieshout et al. Reference Van Lieshout, Schmidt, Robinson, Niccols and Boyle 27 analyzed the association of maternal pre-pregnancy body mass index (BMI) with offspring temperament and behavior in children aged 1 year and 2 years, respectively. In their analysis, maternal BMI was not found to be related with any temperamental component, measured using the Toddler Temperament Scale, nor with internalizing behavior. However, a higher maternal BMI was found to be positively associated with elevated externalizing problems at age 2.Reference Van Lieshout, Schmidt, Robinson, Niccols and Boyle 27 Higher maternal pre-pregnancy BMI was also predictive of a high degree of distress and inattentionReference Dallman, Pecoraro and Akana 28 – Reference Rodriguez, Miettunen and Henriksen 30 and a tendency for child aggressive behavior.Reference Antoniou, Fowler and Reed 31
To the best of our knowledge, there are no studies on the relationship between maternal pre-pregnancy weight and twins’ temperament. In the present study, we used maternal pre-pregnancy BMI as a proxy of the shared prenatal environment to evaluate its influence on infants’ temperament in MZ and DZ twins. A deeper understanding of this association could help preventing the development of difficult temperament in children, as well as antisocial behavior later in life, by informing prospective mothers on the possible effects of overweight and obesity on children’s psychopathology.
Method
Study population
The Twins and Multiple Births Association Heritability Study (TAMBAHS) is a volunteer-based study focusing on the development of twins from birth until 5 years of age. For the present study, mothers of twins aged 0–18 months at the time of the survey were identified. Between July 2008 and May 2010, 417 mothers completed the study’s self-assessed questionnaire on their twins’ temperament. For the determination of the twins’ zygosity, the adapted version of Goldsmith’s zygosity questionnaire was used.Reference Goldsmith 32 This adapted questionnaire, as a method of assigning zygosity, has been validated against determination by identity of polymorphic DNA markers and has reached accuracy in verifying zygosity in 95% of cases.Reference Price, Freeman and Craig 33 In total, 834 twins were included in the analyses; 188 MZ male twins, 188 MZ female twins, 120 DZ male twins, 118 DZ female twins and 220 opposite-sex twins.
Maternal and twins’ characteristics
Pre-pregnancy BMI (expressed as kg/m2) was based on maternal self-report of weight and height and introduced as a continuous variable in the linear regression analysis. Gestational age (measured in completed weeks of gestation), level of education (high school diploma or less, college/professional education and university degree), employment status (housekeeper or unemployed, working part-time, working full-time and others) and smoking (before, during, after pregnancy; yes/no) were noted for mothers; age (in months), sex and birthweight (in grams) were noted for all twins.
Temperament
Infant temperament was assessed using the revised Infant Behavior Questionnaire (IBQ-R).Reference Gartstein and Rothbart 34 The parents were asked to report on a seven-point Likert-type scale the relative frequency of occurrence of specified infant reactions to concrete situations during the previous 7 days. The scale ranged from 1 to 7 (never, very rarely, less than half the times, about half the times, more than half the times, almost always, always and does not apply). The IBQ-R consists of 14 scales. For the purposes of this study, we used only three dimensions of temperament: (1) activity level, which consists of items examining the twins’ movement of arms and legs, squirming and locomotor activity; (2) distress to limitations, which consists of items looking into twins’ fussing, crying or showing distress while (a) in a confining place or position; (b) involved in caretaking activities; (c) unable to perform a desired action; (3) duration of orienting, which consists of items on the twins’ attention to and/or interaction with a single object for extended periods of time. Reliability, convergent validity and relative stability have been demonstrated for the IBQ-R.Reference Bridges, Palmer, Morales, Hurtado and Tsai 35 , Reference Worobey 36 The internal consistency for the IBQ-R items was high with the Cronbach’s α, ranging from 0.81 to 0.90. Consistency between parent report on the IBQ-R and indicators of temperament based on home and laboratory observations has been demonstrated.Reference Schaughency and Fagot 37
Statistical analysis
Mean and standard deviation (s.d.) or median and interquartile range for each continuous variable were calculated as appropriate, stratified by zygosity group. Intrapair twin correlations for each variable, subdivided into MZ and DZ twins, were calculated by using Pearson’s (r) and Spearman’sρ coefficient statistics where appropriate.
The association between maternal pre-pregnancy BMI and each temperamental dimension was analyzed using a multilevel mixed-methods linear regression, in which a random-effect model was added to the default fixed-effects. The intercept of each twin pair was modeled as a function of the population intercept plus a unique contribution of the twin pair, as thoroughly explained by Carlin et al. Reference Carlin, Gurrin, Sterne, Morley and Dwyer 38 and summarized by the following equation:
in which Y ij represents the outcome (i.e., the specific temperamental dimension’ s score) of the jth twin in the ith pair. In addition to the linear regression model, a pair-specific error term (α i ) allows a random shift of the intercept. Regression coefficients (βs) were used as a measure of change on the temperamental dimensions’ scores by a unit change in the mother’s BMI.
Twins’ age, gender, birthweight and gestational age, and maternal age, smoking (before, during and after pregnancy), level of education and employment status were adjusted for in the analysis.
All analyses were performed using STATA v.14. 39
Results
Descriptive statistics as well as Pearson and Spearman correlations between each covariate and the three temperament dimensions stratified by zygosity are shown in Table 1. Mothers’ age was 33.47 years (4.22) for mothers of MZ and 34.65 years (4.05) for mothers of DZ twins. Mothers’ BMI mean score was 23.40 (4.56) for mothers of MZ and 23.46 (5.40) for mothers of DZ, respectively. For MZ and DZ twins, respectively, the median [interquartile range (IQR)] for gestational age was 36 weeks (2.93) and 37 weeks (3.71); for birthweight it was 2438.06 g (793.78) and 2590 g (708.74). MZ and DZ twins were significantly different with respect to gestational age (P=0.0001), birthweight (P<0.0001) and maternal age (P=0.0001).
Table 1 Means/frequencies, standard deviations/percentages and Pearson correlations with the three temperament scales for each covariate, stratified by zygosity

§ Median (IQR) shown.
r a Pearson/Spearman correlation with activity level.
r b Pearson/Spearman correlation with distress to limitations.
r c Pearson/Spearman correlation with duration of orienting.
*P<0.05; **P<0.001; ***P<0.0001.
Means of temperamental dimensions and intrapair twin correlations for MZ and DZ twins are shown in Table 2. For activity level, MZ correlation was r=0.75 and the DZ correlation was r=0.45. For distress to limitations, MZ and DZ correlations were r= 0.83 and r=0.56, respectively. For duration of orienting, MZ and DZ correlations were r=0.94 and r=0.85, respectively.
Table 2 Descriptive statistics of temperament scales for monozygotic and dizygotic twin pairs
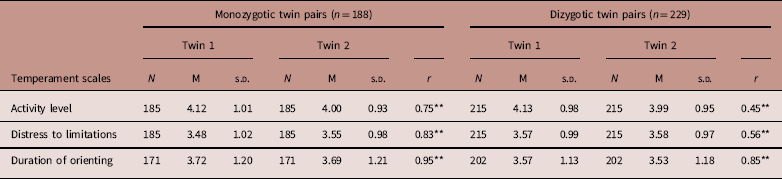
N: Number of twins; M: Mean; r: Within-twin correlations.
Twin 1 is the first born.
Twin 2 is the second born.
*P<0.05; **P<0.001.
Linear regressions
Table 3 presents the results of the linear regressions. In the unadjusted analyses, we found a small decrease in the DZ twins’ distress to limitations score for every unit increase of mother’s pre-pregnancy BMI (β=−0.03; [95% confidence intervals (CI): −0.050, −0.001]; P=0.042). This association was not substantially affected after important confounders were adjusted for (β=−0.04; [95% CI: −0.065, −0.013]; P=0.003). Similar results were obtained when analyzing the MZ and DZ twins combined (results not shown). No association remained statistically significant after controlling for multiple testing: the statistically significance cut-off was set at α=(0.05/18)=0.0028. The normality of residuals was checked through normal quantile plots and confirmed for all regression analyses (results not shown).
Table 3 Linear regressions for the temperamental scales stratified by zygosity based on maternal BMI

SE: standard error of β.
^Adjusted for twins’ age, sex, mother’s age, level of education, employment status and smoking (before, during and after pregnancy).
§ P⩽0.05; *P⩽0.0028.
Discussion
The aim of this study was to examine the relationship between maternal pre-pregnancy weight and infants’ temperament, measured through three dimensions of the revised version of the IBQ: activity level, distress to limitation and duration of orienting. A statistically significant, negative association between maternal pre-pregnancy weight and distress to limitation in DZ twins initially emerged from the present analysis. However, the relative P-value failed to reach the critical level of significance after controlling for multiple testing. Although the Bonferroni method for multiple testing adjustment has been criticized and could be considered excessively conservative in this case,Reference Streiner and Norman 40 the 95% CI clearly shows that the true value of β is close to null, falling between −0.065 and −0.013. This means that for a 1-unit increase in maternal pre-pregnancy BMI, scores in distress to limitation would decrease on average by 0.04 points, on a 1–7-point scale. Consequently, the beneficial effect of a high maternal BMI on children’s temperament we observed is certainly not enough to suggest that a higher maternal pre-pregnancy weight could be beneficial, especially in light of the many negative effects overweight and obesity have on perinatal outcomes and maternal and offspring’s health.Reference Papachatzi, Dimitriou, Dimitropoulos and Vantarakis 41
A large part of the literature suggests that the risk of cognitive and behavioral problems is associated with adverse intrauterine factors.Reference Rodriguez 29 , Reference Rodriguez, Miettunen and Henriksen 30 , Reference Deardorff, Smith, Petito, Kim and Abrams 42 – Reference Thapar, Fowler and Rice 45 Maternal pre-pregnancy overweight or obesity was associated with symptoms of attention-deficit/hyperactivity disorder (ADHD) in school-aged childrenReference Rodriguez, Miettunen and Henriksen 30 and in child with attention and behavioral problems.Reference Rodriguez 29 , Reference Deardorff, Smith, Petito, Kim and Abrams 42 Furthermore, a UK-based, longitudinal study analyzed the influence of maternal pre-pregnancy BMI on children’s cognitive ability, reporting a statistically significant, negative association.Reference Basatemur, Gardiner and Williams 46 However, the effect size was as limited as the one reported in the present study, with a 10-point increase in maternal BMI being associated with a 1/10 s.d. decrease in cognitive performance at age 7 years. To the best of our knowledge, only one study has analyzed the possible influence of maternal pre-pregnancy BMI on singletons’ temperament.Reference Van Lieshout, Schmidt, Robinson, Niccols and Boyle 27 In spite of important methodological differences, such as the specific questionnaire usedReference Gartstein and Rothbart 34 , Reference Oberklaid, Prior, Golvan, Clements and Williamson 47 , Reference Carey and McDevitt 48 and the population of interest (i.e., singletons v. twins), there is consistency in the results between the present and Van Lieshout’s study. Specifically, they reported that temperament at 1 year of age was not associated with maternal pre-pregnancy BMI. Of note, however, is that measures of externalizing behavior on the same population were found to be significantly associated with maternal pre-pregnancy weight. Another study, which analyzed children’s behavior development and cognition in two separate, large cohorts in the United Kingdom and the Netherlands, found no consistent evidence of perinatal influence on these outcomes.Reference Brion, Zeegers and Jaddoe 49 The strong association was observed with children’s IQ at 8 years, while outcomes in younger children were not statistically significant after adjusting for critical confounders nor was repeated in both cohorts. Overall, most of the clinically significant results are reported in studies analyzing older children as opposed to infants and pre-school children, suggesting that the effect of maternal pre-pregnancy BMI may manifest later in children’s development.
The biggest strength of the present study is that the population analyzed is a cohort of young twins. Analyzing twins instead of singletons, automatically controls for genetic (in MZ twins) and common environmental factors (in both MZ and DZ twins). It has been shown previouslyReference Emde and Hewitt 14 that temperament, as well as cognitive and behavioral development, has substantial genetic and environmental influences; therefore not having a genetically informed study design could be an important limitation of all the studies cited above, limiting the confidence in their significant results. Furthermore, the pre-pregnancy environment experienced by singletons may not be comparable with that experienced by twins. In fact, multiple pregnancies are often characterized by nutrient and space scarcity, which may lead to discordant growth or more serious, pathological complications, such as the twin-to-twin transfusion syndrome.Reference Marceau, McMaster and Smith 23 This implies that twins, and especially MZ twins, might be more susceptible to challenging intrauterine environment that might influence their future temperament. As far as we know, only one study explored the association between maternal pre-pregnancy weight and twins’ behavior,Reference Antoniou, Fowler and Reed 31 finding a tendency for children of overweight and obese mothers toward clinically aggressive and externalizing behaviors. It is therefore essential to analyze further the influence of perinatal factors on twins’ psychopathological development.
A vast part of the literature focuses on children’s cognitive and behavioral development as opposed to infants’ temperament, limiting the comparability and understanding of the process. Various suggestions have been put forward for the explanation of the relation between temperament and psychopathology. Of the models suggested, one poses that temperament can be considered as a spectrum, or a common cause model, with normal and abnormal falling at different points on the same continuum. In essence, this model considers temperament to be a sub-clinical manifestation of psychopathology, with shared etiological determinants.Reference Shiner and Caspi 50 The results of the present study suggest, however, that some of the etiological factors important in emotional/behavioral problems, including maternal pre-pregnancy BMI, may not be important for specific temperamental dimensions. This suggests that temperament is not simply a manifestation of psychopathology with a shared etiology. Shiner and CaspiReference Shiner and Caspi 50 have argued that it is implausible that complex behavior such as child psychopathology is the simple product of one or two temperamental factors. Instead, these temperamental factors likely interact with each other and with other variables such that the “true” impact of temperament is larger than the effect of the individual temperamental factors themselves.Reference Shiner and Caspi 50 Evidence for this type of interaction of temperament with other etiological factors has been provided by Owens et al.,Reference Owens, Shaw and Vondra 51 who found that high levels of emotionality in children are linked to lower levels of responsiveness in mothers, which, in turn, may compromise the establishment of a secure attachment relationship. The lack of a secure attachment, then, could further enhance the risk of the development of internalizing/externalizing psychopathology.Reference Fearon, Bakermans-Kranenburg, van Ijzendoorn, Lapsley and Roisman 52
The results of this study should be interpreted in light of some limitations. There was no data available to assess the psychopathology of mothers and their soothing practices toward their twins. Maternal psychopathology is considered a risk factor for obesity and internalizing/externalizing problem behaviors, and it may have an impact on maternal perception of offspring’s behavior and temperament.Reference Durbin and Wilson 53 – Reference Madigan, Oatley and Racine 57 Furthermore, we could not control for parental practices, which might as well be related to infants’ temperament.Reference Prady, Kiernan, Fairley, Wilson and Wright 58 We had no data regarding breastfeeding and maternal Type II diabetes mellitus, which have been found to be associated with children’s temperament and behavior.Reference Ornoy 59 , Reference Shelton, Collishaw, Rice, Harold and Thapar 60 Full control for the familiar socioeconomic status (SES) was not possible, since we lacked the data regarding parental income. Nonetheless, maternal level of education and employment status are considered the other two core components of SES 61 and can provide a reliable indication of the familiar situation. Additionally, we were not able to account for the presence of other children in the family, which might have affected maternal ratings. Although previous studies did not observe an association between chorionicity and children’s psychopathology,Reference Marceau, McMaster and Smith 23 we cannot exclude an uncontrolled effect of chorion type on our results. Finally, although self-reported height and weight are considered accurate enough and widely used in epidemiological studies, it was suggested that overweight and obese women are more likely to under-report their pre-pregnancy weight.Reference Russell, Gillespie, Satya and Gaudet 62 Consequently, we cannot rule out an effect of biased reports on results.
In sum, the results of this study provide evidence that maternal pre-pregnancy BMI, which has been suggested as an important factor in emotional/behavioral problems, is not related with infants’ temperament. These findings suggest that temperament is a complex constellation of characteristics with varying influences, as opposed to a unified, biologically driven system, as previously suggested. Additional studies are needed for elucidating the developmental paths of infant temperament and should consider not only maternal pre-pregnancy BMI but also maternal diet and physical health before and during pregnancy, as well as maternal temperament and psychopathology.
Acknowledgments
The authors wish to thank all participating families.
Financial support
The study was supported by the University of Birmingham (grant number: GAS1168).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the University of Birmingham Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study by the University of Birmingham Ethics Committee.





