Introduction
Obesity and its attendant medical costs afflict at least 35% of the U.S. population,Reference Ogden, Carroll, Kit and Flegal 1 and about one-third of pregnant women are described as clinically obese.Reference King 2 Maternal obesity can lead to severe health complications to the mother and immediate and possible long-term health consequences for her children and grandchildren.Reference Kliegman and Gross 3 , Reference Roseboom and Watson 4 In addition, certain neurological diseases, for example depression, anxiety, attention deficit hyperactivity disorder and autism spectrum disorders (ASD), have been linked to maternal metabolic state.Reference Krakowiak, Walker and Bremer 5 – Reference Lyall, Munger, O’Reilly, Santangelo and Ascherio 9 Maternal diet-induced obesity in both humans and laboratory animals has been linked to epigenetic alterations in key neural genes.Reference Aagaard-Tillery, Grove and Bishop 10 – Reference Yang, Liang and Rogers 16
Past rodent studies suggest that a maternal high-fat diet (HFD) can program later metabolic and neurobehavioral disruptions in adult offspring. One study suggests that rat offspring from dams fed a HFD demonstrate reduced body weight and growth, delayed physical maturation and suppression of physiological reflexes.Reference Mendes-da-Silva, Giriko and Mennitti 17 Another study with rats indicates that a maternal HFD alters the expression of glucorticoid receptor (Gr) and downstream inflammatory genes in the hippocampus and amygdala and correspondingly decreases anxiety-like behaviors in adolescent offspring.Reference Sasaki, de Vega, Sivanathan, St-Cyr and McGowan 18 Rat offspring derived from dams fed a HFD appear to be hypoinsulinaemic and hypoleptinaemic at birth, but by adulthood, are more obese, and show increased concentrations of insulin and leptin compared with controls.Reference Howie, Sloboda, Kamal and Vickers 19 Perinatal exposure to a maternal HFD may also speed up the onset of puberty in female rats.Reference Sloboda, Howie, Pleasants, Gluckman and Vickers 20 In mice, a maternal HFD may increase body length and disturb glucose homeostasis across multiple generations.Reference Dunn and Bale 21
Additional studies have examined whether other nutrients may be used to mitigate the effects of a HFD, and whether sons and daughters demonstrate differing vulnerability to this imposed maternal diet. Mice offspring born to dams fed a HFD go on to show increased body weight gain, increased dietary fat preference in the case of male offspring, changes in central nervous system (CNS) gene expression, and global hypomethylation in the prefrontal cortex.Reference Carlin, George and Reyes 22 However, concurrent maternal supplementation with a methyl-donor enriched diet combats these adverse effects. Male offspring born to rats fed a HFD during pregnancy and lactation and before conception show no anxiogenic behaviors, as determined by the elevated plus maze (EPM) (described below) but exhibit altered responses in the open field test, including increased zone entries and reduced thigmotaxis behavior (i.e. an organism’s tendency to approach and seek contact with another object or fellow organism).Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 23 These offspring also demonstrate reduced approach behavior and learning impairments, but sons born to mothers initially fed a HFD before conception, and then switched during gestation and/or lactation to a control diet, demonstrate improved performance, suggesting that there might be critical windows of time where maternal dietary intervention strategies can be employed to reverse the harmful effects of a HFD.
Many of the above studies employed laboratory mice and rats, which in some cases were inbred. Although such studies are helpful in elucidating how a maternal HFD can affect offspring outcomes, to recapitulate most human societies, it would be helpful to study such a maternal diet in an outbred monogamous animal model where both parents help raise the young. The oldfield mouse (Peromyscus polionotus) is monogamous and biparental,Reference Margulis 24 , Reference Vrana, Shorter and Szalai 25 with the social bond between mated pairs essential in pup rearing (as we have shown for the related monogamous Peromyscus californicus).Reference Rosenfeld, Johnson, Ellersieck and Roberts 26 Their social structure is thus similar to most human societies. Consequently, we used oldfield mice to examine further how a maternal HFD may affect, in a sex-dependent manner, later offspring behavioral and metabolic parameters. Another important strength of this study is that, in the same group of animals, the effects of a maternal HFD on offspring behavioral and metabolic patterns were examined to determine if this diet simultaneously impacts the brain and metabolic organs. It should also be noted that the oldfield mouse model has been used widely in genetic, environmental, hormonal and toxicological studies.Reference Vrana, Shorter and Szalai 25 , Reference Carmon, Williams and Golley 27 – Reference Wolfe, Esher, Robinson and Yarbrough 42 Moreover, this rodent model and other Peromyscus species have also been proposed to be complementary animal models to understand ASD in humans.Reference Shorter, Owen and Anderson 34 , Reference O’Neill, Vrana and Rosenfeld 43 – Reference Tanimura, King, Williams and Lewis 46 In the current studies, P0 females were exposed to a maternal HFD or control diet before conception and throughout lactation. The effects of a maternal HFD on later adult F1 male and female offspring behaviors, spatial learning and memory, ability to extinguish a learned response, anxiety-like and exploratory behaviors, voluntary physical activity and metabolic parameters were then assessed.
Materials and methods
Animal husbandry
Outbred adult (60–90 days of age) founder oldfield female and male mice (Peromyscus polionotus subgriseus, PO stock) females and males, free of common rodent pathogens, were obtained from the Peromyscus Genetic Stock Center (PGSC) at the University of South Carolina (Columbia, SC, USA), and placed in quarantine for a minimum of 8 weeks to ensure that they did not carry any transmittable and zoonotic diseases. From the time ancestors were originally captured in the Ocala National Forest in 1952, P. polionotus subgriseus captive stocks have been bred by the PGSC to maintain their outbred status. All experiments were approved by University of Missouri Animal Care and Use Committee (Protocol #7753) and performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. About 2 weeks before breeding, 8–12 weeks of age females were randomly assigned to receive one of two diets: Control (7.2% fat, TD.140790, Envigo, Madison, WI, USA) or HFD (22.2% fat, TD.130957, Envigo). Nutrient composition of each diet is listed in Supplementary Table 1. We weighed three dams on each of the diets at the time they were paired to establish that they were approximately the same weight at the time the diets were initiated. They were then weighed 1 and 2 weeks after being on their respective diets. After 2 weeks on the diets, females were then singly paired with males and continued on the diets until offspring were weaned at 30 days of age, the typical age for this species. Peromyscus species generally do not display copulatory plugs. However, we did not notice any preterm delivery, as has been reported in women consuming a HFD,Reference Englund-Ogge, Brantsaeter and Sengpiel 47 or prolonged gestation for the two groups. After the females were paired to males and throughout the time they were nursing, they were minimally handled to avoid any stress that might cause them to abort, as this had been a concern while establishing the colony. Thus, we were not able to obtain any additional data on the dams.
Before being paired to females, all males were maintained on the control diet to avoid the HFD influencing their germ cell development and reproductive tract secretions, which could impact offspring metabolic and other outcomes.Reference Binder, Hannan and Gardner 48 – Reference Binder, Beard and Kaitu’u-Lino 53 As this species is monogamous and biparental, the males were maintained with their breeding partner and offspring throughout the neonatal period.
The average litter size for P. polionotus is three to four pups.Reference Kirkland and Layne 54 F1 male and female offspring were weaned and placed on the control diet. Male and female offspring from the control and maternal HFD were tested at the same ages and order of tests. The total litters and number of F1 male and female offspring used in the experiments below are listed in Supplementary Table 2. There were no differences in sex ratio for either diet group. As shown in Supplementary Table 2, for reasons that are not clear, in the same amount of time and with the same number of original females placed in each of the groups, there were more litter generated from dams on the HFD than the control diet, which is a standard gestational/lactational diet for rodents. Even so, the number of litters and offspring for both groups is on par and in some cases greater than total replicates tested in previous studies, which all show that a maternal HFD can affect offspring outcomes.Reference Mendes-da-Silva, Giriko and Mennitti 17 – Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 23 , Reference Rosenfeld, Grimm and Livingston 55 , Reference Wu, Deng and Li 56 The same group of animals was used in all tests detailed below.
Barnes and reverse Barnes maze
When F1 offspring reached ~90 days of age, their spatial learning and memory was tested in the Barnes maze. This test was performed as described previously for deer mice (P. maniculatus bairdii) and California mice (P. californicus).Reference Jasarevic, Williams, Roberts, Geary and Rosenfeld 57 – Reference Williams, Jasarevic and Vandas 60 The maze was comprised of a circular platform with 12 escape holes that were placed every 30°.Reference Rosenfeld and Ferguson 61 To help guide the animal to the correct escape hole, four geometric shapes (triangle, square, circle and star) were placed 10 cm from the platform and evenly spaced (at 90° intervals) along the maze wall. These served as intra-maze visual cues to help guide the animal to the correct escape hole. Before the behavioral testing, each animal was randomly assigned an escape hole that remained the same over the 5 days test period. To encourage the animal to enter the escape hole, a bright light (1200 lx v. testing room light 400 lx) was placed over the maze to serve as a mildly aversive stimulus. The animals were habituated to the testing room conditions for 30 min. They were then placed in the center of the maze and provided 300 s to locate the correct escape hole. If they did not find it on the 1st trial day, they were gently guided to it. Each trial was recorded with a Canon Vixia HF HD hand held camcorder (Canon, Melville, NY, USA). After the animals were rested for 1 week, they were retested with a reverse Barnes maze approach, where there was random reassignment of a different correct escape hole. The amount of time it took the animals to learn the new escape hole was then measured.
The initial and reverse Barnes maze behavioral trials were analyzed with the ANY-maze (Stoetling Co., Wood Dale, IL, USA) software analysis system for behavioral analysis. Indices that were measured with this program included time to enter the correct escape hole (latency), velocity, distance traveled, amount of time in the correct target zone and amount of time in the incorrect target zone. In addition, this program tracks the path the animals take to get from the center of the maze to the correct escape hole, which results in three main spatial strategies. A serial search strategy (Coded 1) was defined as continual search of consecutive holes in a clockwise or counterclockwise manner. A random search strategy (Coded 2) entailed the animal randomly searching non-escape holes while also crossing the center of the maze twice or more. The direct search strategy (Coded 3), which is the most efficient, was characterized with the animal entering the correct escape hole without crossing the center of the maze and entering no more than three incorrect holes.
EPM
The EPM measures exploratory and anxiety-like behaviors. This procedure was performed in the week between completion of the Barnes maze testing and initiation of the reverse Barnes maze testing, and the procedure was done as described previously.Reference Jasarevic, Williams and Vandas 58 – Reference Williams, Jasarevic and Vandas 60 , Reference Fountain, Mao and Whyte 62 The EPM is arranged in a plus configuration and includes two opposite open arms (30 cm), a central platform region (5×5 cm), and two opposite closed arms (30 cm). Each animal was placed in the center of the maze and permitted to explore it for 300 s. Each trial was recorded with a Canon Vixia HF HD hand held camcorder (Canon). The video trials were then analyzed with the Observer Version 11 software (Noldus Technologies, Leesburg, VA, USA). Parameters measured include number of entries into the open and closed arms, duration of time spent in the open and closed arms and center, head dipping, which suggests exploratory, non-anxious behavior and rearing.
Voluntary wheel running
Voluntary wheel running was measured after the reverse Barnes maze procedure was performed.Reference Johnson, Painter and Javurek 63 Metal exercise wheels (Kaytee, Chilton, WI, USA) with a diameter of 5.75 inches that were connected to a bicycle computer (Sigma Sport BC12.12, Sigma Sport USA LLC; St. Charles, IL, USA) were used to measure total distance traveled, average speed, maximum speed and total time spent running on the wheels for the 5-day trial period.
Indirect calorimetric testing
After completion of the above tests, adult (19–31-week old) animals were tested in the Promethion continuous measurement indirect calorimetry system (Sable Systems International, Las Vegas, NV, USA) for 3 days as described previously.Reference Johnson, Painter and Javurek 63 The reason for this varying age range for this and subsequent experiments is that we sought to test the animals at a range of ages to determine whether the changes were stable over time. Moreover, this equipment is shared and heavily used, and, thus, it necessitates animals being tested in groups, which is another reason for the varying ages at the time of testing. Data were divided into 12 h light and 12 h dark cycles. Parameters that were measured include energy expenditure (EE), respiratory quotient (RQ) from oxygen consumption and CO2 production, and activity in the home cage as measured by beam breaks (X – vertical, Y – horizontal and Z-rearing), food and water intake.
Echo magnetic resonance imaging (EchoMRI)
After the indirect calorimetric testing was completed, the animals were tested in the EchoMRI-1100 (EchoMRI LLC, Houston, TX, USA) to measure body composition as described previously.Reference Johnson, Painter and Javurek 63 Variables measured in a rapid and non-invasive manner included total and lean fat, free water and total water mass.
Serum hormone analyses
After completion of the echoMRI testing, the animals were fasted overnight. They were then humanely euthanized the next morning at ~8.30 am, and cardiac blood collected. The blood was centrifuged for 15 min at 7500 g in a Hettich Zentrisugen centrifuge (Hettich Lab Technology, Beverly, MA, USA). The serum fraction was then collected and stored at −80°C until metabolite and hormone analyses were performed. Serum glucose was measured with a commercial clinical chemistry analyzer (Beckman-Coulter AU680; Beckman-Coulter, Brea, CA, USA) and automated, commercially available assay (Beckman-Coulter). Plasma insulin (Crystal Chem, Catalog # 90080; Downers, Grove, IL, USA) and leptin (Crystal Chem, Catalog # 90030) concentrations were analyzed according to the manufacturer’s instructions for each of these ELISA kits, but without any serum dilution.
Statistics
SAS version 9.2 software analyses software (SAS Institute, Cary, NC, USA) was employed for these analyses. Unless otherwise stated, the reported data are based on mean±s.e.m. For all experiments, we first considered whether there were offspring and interactions of offspring×maternal sex or day effects. In those cases, were there were no main or interaction offspring effects, both sexes were considered together.
Maternal periconception body weight
As the maternal periconception body weight was collected at three time-points: at the time of being placed on the control or HFD, 1 and then 2 weeks later, these data were analyzed by a repeated measure ANOVA approach.
Barnes and reverse Barnes maze
Continuous random variables assessed in the Barnes maze, including distance traveled, velocity, error rate, were analyzed as a split plot in space and time.Reference Steel 64 The linear statistical model contained the fixed effects of diet, sex, day and all possible interactions with diet, sex and day. To determine whether there were litter effects, source (dam×male) within diet was used as the denominator of F for diet, source within day×sex was used as the denominator for sex and interaction of diet×sex, source within day was used as the denominator of F for day and the remaining interaction used the residual mean square as the denominator of F. Mean differences in body weight were determined by using Fisher’s protected least significant difference (LSD).
Latency data were analyzed by using the PROC LIFETEST and Proportional Hazard Ratio (PROC PHREG) functions in the SAS version 9.2 software analyses. These analyses adjust for right-censoring (defined here as not locating the escape box within the allotted time of 300 s) while still accommodating the study design of 300 s/trial. Data are reported as a hazard ratio that signifies the odds of a subject in a treatment group locating the correct escape hole compared with the other groups tested. A significant result indicates the odds are not 1:1. A result >1 indicates the test group was more likely to locate the correct escape hole than all other groups tested. A result <1 indicates that the treatment group is less likely to locate the correct escape hole compared with the other study groups. The litter was used as the denominator of F for the effects of maternal diet, offspring sex and test day, and potential interactions between maternal diet, offspring sex and test day. Latency data are reported as the mean, 95% lower and upper confidence limit.
Search strategy data were analyzed by using a repeated measurement design with PROC GLIMMIX and SAS version 9.2 software analyses (SAS Institute). This analysis used a cumulative logit link and a multinomial distribution with all three search strategies included in this approach. As this initial analysis indicated a significant three-way interaction between maternal diet×sex×day, another cumulative logit analysis for each day was performed, where maternal diet, sex and maternal diet×sex interaction were modeled. To analyze the differences further, a third analysis on search strategy was performed on which the two less efficient strategies (1 and 2) were combined and compared against the more efficient search strategy (3), thereby resulting in a binomial distribution. The PROC GLIMMIX was again used where the model contrasted maternal diet, sex, maternal diet×sex effects for each day with a logit link. The differences between the least square means were based on average logits. Tabled data were converted to probabilities, which is the probability of a treatment group using one of the less efficient search strategies compared with the most efficient or direct search strategy.
Elevated plus maze
The amount of total time spent in the open and closed arms and center, as well as total number of arm entries, average velocity, total distance traveled, number of times engaged in head dipping and rearing were analyzed by a split plot design, as described above. The main variables included the effects of maternal diet, sex and maternal diet×sex.
Indirect calorimetric testing
The data were analyzed as a repeated measurement analysis in which the main plot contained the effects of the two maternal diets and two offspring sexes. The denominator of F for the main plot was litter within maternal diet and offspring sex. The subplot contained the time series of both day and cycle. The day and cycle were factorial arranged in which the cycle contained two cycles (dark and light) and day contained the two days in which animals were measured in this unit. The subplot effect of day and cycle and day×cycle and the interactions of day and cycle with the main plot effect were tested using litter within maternal diet, offspring sex, day and cycle as the denominator of F. Fisher’s protected LSD was tested if the overall of F was significant. In addition, as the animals were tested from 19 to 31 weeks of age, we divided the animals up into two age groups: 19 to 22 weeks and 23 to 31 weeks. The reason for these two groupings is that it allowed sufficient number of replicates in each of the maternal diets and sexes to be examined. We then considered for each of the categories measured with this equipment, the main effects of age and all possible interactions of age with maternal diet, sex, day and cycle.
EchoMRI
The data were analyzed as a complete randomized design (CRD) in which treatments were arranged as a two by two factorial (two maternal diets and two offspring sexes). As multiple pups came from the same litter, dam within maternal diet and offspring sex was used as the denominator of F. If the overall F was significant, then differences were determined using Fisher’s protected LSD.
Voluntary wheel running
The data were analyzed as a repeated measurement design in which the main plot contained maternal diet and offspring sex and maternal diet×offspring sex in a two by two factorial design. The subplot contained day and all possible interactions with the main plot effect. The denominator of F for the main plot was dam within maternal diet and offspring sex. The denominator of F for the subplot effects was dam within maternal diet, offspring sex and day. Mean differences were determined using Fisher’s protected LSD when the overall F test was significant.
Serum hormone data
For these data, the litter was considered the experimental unit. However, only on rare occasion, two animals originated from the same litter. The data were analyzed as a simple two by two factorial arrangement (two maternal diets and two sexes) considering the design as a CRD. If the overall F was significant, a Fisher’s protected LSD was performed.
Results
Maternal periconception body weight
For the three time-points measured (day placed on diet, 1, and then 2 weeks afterwards), there was no effect of diet on maternal periconception body weight (Supplementary Table 4).
Barnes and reverse Barnes maze
There was a significant difference between offspring of the different maternal diets for the initial and reverse Barnes maze tests. Compared with controls, HFD offspring sniffed fewer incorrect holes, which equates to a decreased error rate, in the initial Barnes maze (Fig. 1a, P=0.002). However, the control group experienced a significant improvement between the initial and reverse maze (P=0.0002), whereas the HFD group did not. For distance traveled, there was a significant interaction between the two Barnes maze trials and maternal diet, as well as the two trials and sex (P=0.03 and 0.05, respectively). In the initial Barnes maze test, control animals traveled more distance than HFD individuals (Fig. 1b, P=0.03). Control individuals traveled more distance in the initial compared with the reverse Barnes maze (P=0.02). Females traveled greater distance in the initial compared with the reverse Barnes maze (Supplementary Fig. 1, P=0.01). For velocity, the HFD group, but not the control group, significantly increased their locomotion speed in the reverse condition compared with the initial Barnes maze (Fig. 1c, P=0.0002).

Fig. 1 Sniffing incorrect holes, distance traveled, velocity in the Barnes and reverse Barnes maze. (a) Sniffing incorrect holes. (b) Distance traveled. (c) Velocity. Significant differences are designated with brackets between the values that differ and P-value above the brackets.
For latency in both Barnes trials, there was a significant interaction between maternal diet and sex (P=0.0001). Although no differences were evident in females between the two maternal diet groups, F1 males in the HFD group demonstrated increased likelihood of solving the maze in the allotted time compared with control males (Fig. 2, P=0.0001). Within the control group, female offspring were more likely to solve the maze than their male counterparts (P=0.0001). These same sex differences were not evident in the HFD group. There were no differences in search strategy based on maternal diet, sex or Barnes v. reverse Barnes maze tests (Supplementary Fig. 2).

Fig. 2 Overall likelihood for females and males in each treatment group to locate the correct escape hole in the Barnes and reverse Barnes maze tests (latency). An increased ratio indicates shorter latency. The upper, middle and lower bars represent the ratio of locating the correct escape hole at the 95% upper confidence limit, mean, and 95% lower confidence limit, respectively, for each group to locate the escape hole relative to all other animals tested. Significant differences are designated with brackets between the values that differ and P-value above the brackets.
There were also differences based on sex×initial/reverse Barnes maze×day (P=0.05). In the initial Barnes maze, both females and males learned to solve the maze over the 5-day trial period, as evidenced by the increased likelihood of locating the correct escape hole in the allotted time in later compared with the early trials (Supplementary Fig. 3a). Even by day 2, both sexes showed improved performance compared to the initial trial day (P=0.002 for females and P=0.0001 for males). This trajectory continued and became more pronounced in the later trial days (days 4 and 5, P=0.0001). However, the results for the reverse Barnes maze were not as clear (Supplementary Fig. 3b). In females, improved performance was only observed in day 2 v. days 3 and 4 (P=0.02). Male showed increased likelihood of solving the maze on days 4 and 5 compared with day 2 (P=0.003 and 0.01, respectively). In addition, differences were observed between days 3 and 4 (P=0.05).
EPM
For the duration of time spent immobile and mobile, there was a maternal diet×sex interaction (P=0.04 for both categories). F1 adult daughters from dams fed the HFD spent more time immobile and less time mobile compared with F1 control daughters (Fig. 3a, P=0.02). HFD daughters also spent more time immobile and less time mobile that HFD sons (P=0.02). No differences were detected across maternal diet groups for F1 males. The number of entries into the open or closed arms did not vary based on maternal diet or sex (Fig. 3b). Both groups entered the open arms more than the closed arms.

Fig. 3 Elevated Plus Maze results. (a) Time spent immobile and mobile. (b) Entries into open and closed arms. (c) Duration of time spent head dipping. (d) Mean time spent rearing. Significant differences are designated with brackets between the values that differ and P-value above the brackets.
Head dipping, indicative of exploratory behavior, differed based on maternal diet (P=0.05). F1 HFD offspring spent decreased amount of time engaging in this behavior compared with control offspring (Fig. 3c). The mean time spent rearing, which is considered a stereotypical behavior, varied according to maternal diet×sex (P=0.05). F1 HFD males spent greater mean time rearing than control males and F1 HFD females (Fig. 3d, P=0.03 and 0.01, respectively).
Voluntary wheel running
There were no differences in distance traveled or average speed based on maternal diet or offspring sex (Supplementary Fig. 4).
Indirect calorimetry
Total EE, indicative of total energy expended in kcal, did not differ. Resting EE differed based on a maternal diet×circadian cycle as indicated by a significant statistical interaction (P=0.008). During the dark (i.e. rodent active cycle), control individuals demonstrated greater resting EE compared with maternal HFD offspring (Fig. 4, P=0.03). In controls, resting EE was greater during the dark cycle compared with the light cycle (P=0.0001), but such circadian differences were not evident in the HFD group which may indicate that the maternal HFD reduced resting EE during the rodent’s active cycle.

Fig. 4 Average energy expenditure (EE) and food consumption. (a) Average EE for 30 min with the lowest EE (resting EE). (b) Average food consumption for females. (c) Average food consumption for males. Significant differences are designated with brackets between the values that differ and P-value above the bracket.
For resting EE, there was also an age×sex interaction (P=0.03). At 23–31 weeks of age, females trended to show a lower EE than males (P=0.06, Supplementary Fig. 5).
Average food consumption showed significant differences based on maternal diet×sex×cycle interaction effects (P=0.01). During the light cycle, food consumption (both of these offspring groups were maintained on the same control diet) was greater for control females than HFD females (Fig. 4b, P=0.002). No treatment differences though were observed in male offspring (Fig. 4c).
For average food consumption, there were also significant differences based on maternal diet×age×cycle interaction effects (P=0.004). At 19–22 weeks of age, control animals consumed more food during the dark compared with the light cycle (Supplementary Fig. 6, P=0.001). In comparing across age groups, control animals ate more during the light cycle from 23 to 31 weeks compared with 19–22 weeks (P=0.0006). Conversely, control animals ate more during the dark cycle at 19–22 weeks than at 23–31 weeks (P=0.05). At 19–22 weeks of age, control animals ate more during the dark cycle than HFD animals of this age range (P=0.02). At 23–31 weeks of age, control animals ate more during the light compared with the dark cycle (P=0.001). Further, at this same age, control animals ate more during the light cycle than HFD animals (P=0.001).
Several indices measured in the indirect calorimetry suggested that the maternal HFD suppressed voluntary physical activity in resulting adult offspring. The number of times individuals broke the beams on the X, Y, or Z axis (XYZ Breaks) varied based on diet×cycle interaction effects (P=0.02). During the dark cycle, control individuals broke the beams more times than those in the HFD group (Fig. 5a, P=0.003). Both control and HFD individuals broke the beams more times during the dark than the light cycle (P=0.0001). Males in both combined groups broke the XYZ beams more times during the dark cycle than females in both groups (Supplementary Fig. 7, P=0.007). Total distance walked differed based on maternal diet×cycle interaction effects (P=0.01). During the dark cycle, control individuals walked more than those in the HFD group (Fig. 5b, P=0.003). The percentage of time spent walking and remaining still also varied according to maternal diet×cycle (P=0.02 and 0.01, respectively). Control animals walked a greater percentage during the dark cycle than HFD individuals (Fig. 5c, P=0.009). Conversely, HFD individuals spent more time remaining still during the dark cycle than control counterparts (Fig. 5d, P=0.004).

Fig. 5 Voluntary physical activity home-cage setting assessments. (a) X, Y, and Z beam break measurements. (b) Total distance traveled. (c) Percentage of time spent walking. (d) Percentage of time remaining still. Significant differences are designated with brackets between the values that differ and P-value above the bracket.
For these above parameters, only total distance walked was influenced by maternal diet×age×sex. At 19–22 weeks of age, control females tended to walk more than HFD females (Supplementary Fig. 8, P=0.06). At 23–31 weeks of age, control females walked less than control males (P=0.01). At this same age range, control males walked more than HFD males (P=0.02).
The percentage of time spent sleeping was significantly altered by maternal diet×cycle effects (P=0.004). HFD animals spent a greater percentage of time sleeping during the dark cycle than controls (Supplementary Fig. 9a, P=0.0004). Both controls and HFD animals spent a greater percentage of time sleeping during light v. dark cycle (P=0.0001). The total hours spent sleeping was significantly affected by maternal diet×cycle effects (P=0.004). During the dark cycle, HFD offspring slept more than controls (Supplementary Fig. 9b, P=0.0004). Both control and HFD offspring slept more during the light compared with the dark cycle (P=0.0001).
EchoMRI
Adult body weight differed based on maternal diet (P=0.01). Sons and daughters born to dams on the HFD weighed more as adults that those born to dams on the control diet (Fig. 6a). Adult lean tissue was also greater in offspring exposed to a maternal HFD (P=0.002). Control females and males had less lean tissue mass than HFD counterparts (Fig. 6b, P=0.008 and 0.01, respectively).

Fig. 6 Echo magnetic resonance imaging results. (a) Adult body weight. (b) Lean and fat tissue mass. Significant differences are designated with brackets between the values that differ and P-value above the bracket.
Serum metabolites and hormones
No differences were detected based on maternal diet or sex for serum glucose, insulin or leptin concentrations (Table 1).
Table 1 Measurements of serum glucose and metabolic hormone concentrations in offspring
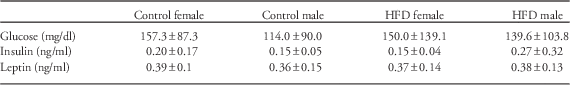
HFD, high-fat diet.
Discussion
The primary goal of this study was to examine how maternal periconception through lactation exposure to a HFD in oldfield mice affected behavioral and metabolic parameters in her adult F1 male and female offspring. Counter to expectations, male offspring exposed to the maternal HFD showed improved spatial learning and memory ability compared with control males. This was evident by the fact that the latter group was more likely to solve the initial and reverse Barnes maze in the allotted time (shorter latency) and thus traveled less distance in the maze. A prior study with Wistar rats found that maternal obesity due to a HFD resulted in learning deficits in F1 offspring.Reference Wu, Deng and Li 56 Analogous results were reported in Sprague-Dawley rat offspring born to dams fed a HFD in that they took longer and swam more in the Morris Water Maze, which also measures spatial learning and memory.Reference Page, Jones and Anday 65 That study also showed that the HFD offspring had gene expression changes in the hippocampus with nerve growth factor (Ngf) and N-methyl-d-aspartate receptor (NMDA) receptor subunit NR2b (Grin2b) decreased but synaptotagmin (Syt1) increased. In mice offspring, a maternal HFD impaired performance in the Barnes maze, increased lipid peroxidation in the hippocampus, decreased dendritic arborization in hippocampal neurons and suppressed brain-derived neurotrophic factor (Bdnf) expression in the hippocampus.Reference Tozuka, Kumon and Wada 66 It is not clear why the current findings differ from those earlier studies. It may relate though to the varying animal models tested. In oldfield mice, which tend to be smaller than most laboratory mice and rats, development of this cognitive domain may benefit from a maternal HFD; whereas, a similar maternal diet in mice and rats may result in adverse effects on later spatial learning and memory. The age at which the animals are tested may also affect the results. Although HFD males may show initial improvements in spatial learning and memory, they could also show premature cognitive decline relative to controls. Another possibility for the conflicting data may be due to variation in the diet composition used in the past studies relative to the current studies. In prior experiments, the fat kcal % ranged from 34.9 to 57.8,Reference Wu, Deng and Li 56 , Reference Page, Jones and Anday 65 , Reference Tozuka, Kumon and Wada 66 whereas in the current work it was 43.8% (Supplementary Table 1). There were also variations across studies in the kcal/g, protein and carbohydrate content in the diets tested. Similar to our experimental design, these above studies exposed the females to the HFD before mating (periconception period) and throughout most if not all of lactation.Reference Wu, Deng and Li 56 , Reference Page, Jones and Anday 65 , Reference Tozuka, Kumon and Wada 66 However, in these studies, the females were exposed to the diet from 4 to 6 weeks before mating, which could lead to varying results. It is increasingly becoming clear that different exposure windows, periconception, gestation and/or lactation, can induce contrasting offspring DOHaD outcomes.Reference Agnoux, Antignac and Simard 67 – Reference Yang, Shen and Cai 70 Thus, future work may need to test this and other behaviors at multiple ages, test diets containing a range of fat content, and examine different exposure windows during these three critical periods.
The two Barnes maze trials also reveal that in the control group, females outperform males, as indicated by increased likelihood of solving the maze. This finding is surprising in light of our prior studies with related Peromyscus cousins. In polygynous deer mice (P. maniculatus bairdii), males showed enhanced spatial learning and memory compared with females.Reference Jasarevic, Williams, Roberts, Geary and Rosenfeld 57 – Reference Jasarevic, Sieli and Twellman 59 In this species, this behavior is considered a sexually selected trait, as sexually mature adult males relay on spatial abilities to locate potential reproductive partners that are widely dispersed throughout the environment. In contrast, this behavior does not confer any advantage and may actually put female deer mice at risk for increased predation. Conversely, monogamous California mice (P. californicus) do not demonstrate any sex differences in spatial navigation.Reference Jasarevic, Williams, Roberts, Geary and Rosenfeld 57 , Reference Williams, Jasarevic and Vandas 60 In oldfield mice, females forage for food for themselves and their offspring, and increased spatial ability might allow them to recall the best food sites. We previously found that female Sprague-Dawley rats demonstrate enhanced spatial learning and memory compared with males.Reference Johnson, Javurek and Painter 71 The combined results suggest that sex-differences in spatial learning and memory may depend on mating strategies and which sex is the primary nutritional provider.
Anxiety-like and exploratory behaviors were assessed with the EPM. Decreased mobility by F1 HFD females in the EPM suggests that they are more anxious, less exploratory than their control counterparts. Decreased exploratory behavior was also evident as both F1 HFD females and males spent less time engaging in head dipping behavior. In contrast, F1 HFD males spent more time rearing, a stereotypical behavior, than F1 control males. Prior rodent studies have shown disparate results as to whether a maternal HFD results in increased anxiety-like behaviors in their offspring.Reference Sasaki, de Vega, Sivanathan, St-Cyr and McGowan 18 , Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 23 , Reference Sasaki, de Vega, St-Cyr, Pan and McGowan 72 – Reference Peleg-Raibstein, Luca and Wolfrum 74 Sex, age at the time of behavioral testing, and different rodent models might account for the conflicting findings. In humans, maternal obesity has been linked with child obesity, increased inflammation and anxiety disorders.Reference Weiss, Dziura and Burgert 75 – Reference Rofey, Kolko and Iosif 79 Besides neural inflammation, a maternal HFD might disrupt anxiety responses via glucocorticoid receptor, gamma-aminobutyric acid, serotonergic and neurotrophin signaling pathways.Reference Sasaki, de Vega, Sivanathan, St-Cyr and McGowan 18 , Reference Sasaki, de Vega, St-Cyr, Pan and McGowan 72 – Reference Peleg-Raibstein, Luca and Wolfrum 74
During the time period assessed, no differences in wheel running were observed between the maternal HFD and control offspring. It has been previously reported that Peromyscus spp., including P. polionotus, run more on running wheels than Microtus species.Reference Dewsbury 80 It is possible that if the running wheel were left in the cages for several months, differences would emerge between the two groups. For instance, HFD offspring might become more habituated and less likely to use the wheels than controls. This possibility is currently being explored. There may also be species and sex-differences in vulnerability to maternal diet changes and usage of voluntary wheels in her offspring. For instance, ad libitum feeding of a maternal HFD to Sprague-Dawley rats resulted in decreased voluntary wheel running in her sons, but increased time spent on them by her daughters.Reference Cunha Fda, Dalle Molle and Portella 81
Although no maternal diet-induced changes in offspring physical activity were observed with the voluntary wheels, dramatic differences were detected with indirect calorimetry testing, which may be a better assessment of general home-cage activity. Several indices, including X, Y and Z beam breaks, percentage and total duration of time spent walking, distance traveled and percentage and total duration of time spent sleeping, strongly indicate that offspring born to dams on the HFD are less active than control counterparts. During the rodent active cycle, control offspring were more likely to move around the cage than HFD offspring. In contrast, sons and daughters exposed to the maternal HFD were more likely to sleep during this time, which is generally considered the height of rodent activity. Correspondingly, controls demonstrated greater EE during the dark cycle than HFD progeny.
Our prior studies indicate that maternal exposure of related California mice (P. californicus) to the endocrine disrupting chemical, bisphenol A (BPA), resulted in analogous findings in terms of decreased voluntary physical activity.Reference Johnson, Painter and Javurek 63 BPA has been proposed to be an obesogen, especially when exposure occurs during the perinatal period.Reference Liu and Peterson 82 – Reference Schug, Janesick, Blumberg and Heindel 84 Thus, it is not surprising that similar findings are observed in progeny of dams exposed to a HFD or BPA. In light of the significant prevalence of obesity during pregnancy, combined with potential endocrine disruptive agents in our environment, future studies should determine if maternal co-exposure to a HFD and BPA exacerbates physical inactivity in resulting offspring.
In sheep, maternal undernutrition is associated with decreased voluntary physical activity by adult offspring.Reference Donovan, Hernandez and Matthews 85 Similar findings were reported in Wistar rat offspring derived from undernourished mothers.Reference Vickers, Breier, McCarthy and Gluckman 86 Moreover, provisioning these offspring with a hypercaloric diet at weaning accentuated the physical inactivity, Fetal growth restriction of offspring derived from viable yellow (A vy /a) mice dams results in later physical inactivity and obesity.Reference Baker, Li, Kohorst and Waterland 87 An epidemiological study with parents and offspring in South India revealed that offspring physical activity significantly correlated with physical activity level of the mother but not the father.Reference Swaminathan, Thomas, Yusuf and Vaz 88
Mechanistically, reduced spontaneous cage activity in the home-cage setting for HFD sons and daughters may be due to disturbances in brain regions governing this behavior, such as the hypothalamus, hippocampus, amygdala, prefrontal cortical region, nucleus accumbens, caudate-putamen, mid-brain, locus coeruleus and pons.Reference Rhodes, Garland and Gammie 89 – Reference Teske, Perez-Leighton, Billington and Kotz 96 A maternal HFD may also lead to changes in neural transcripts modulating voluntary physical activity, including DeltaFosb,Reference Werme, Messer and Olson 97 dopamine receptor and transporter (Drd1-5 and SlcA3),Reference Alyea and Watson 98 – Reference Garland, Schutz and Chappell 104 Bdnf,Reference Kolb, Rezende and Holness 95 , Reference Chen, Jing and Bath 105 , Reference Berchtold, Kesslak, Pike, Adlard and Cotman 106 orexin and orexin receptor (Oxa and Oxr).Reference Teske, Perez-Leighton, Billington and Kotz 96 , Reference Teske, Billington and Kotz 107 – Reference Perez-Leighton, Boland, Teske, Billington and Kotz 111 Future studies will thus be designed to test whether a maternal HFD alters these neural transcripts and brain regions. If so, therapies may be designed to correct these gene expression imbalances.
We found that HFD progeny weighed more than controls, although we did not see any changes in fat content. Earlier and later time-points are currently being examined to elucidate the etiology and potential significance of this phenotypic change. Other animal model and human epidemiological studies suggest that offspring born to obese mothers and those consuming a HFD show a greater preponderance to become obese as well.Reference Gaillard 112 – Reference Zambrano, Ibanez and Martinez-Samayoa 116 Given the body weight differences, we predicted that there would be alterations in serum metabolites and hormones. However, serum glucose, insulin and leptin concentrations in fasted animals did not differ based on maternal diet. A prior study showed that HFD rat offspring demonstrate decreased circulating concentrations of insulin and leptin at birth but by adulthood, these hormones are elevated relative to control, non-obese offspring.Reference Howie, Sloboda, Kamal and Vickers 19 The combined studies suggest that there could also be species and temporal differences as to how a maternal HFD affects these factors. Other metabolic hormones, including orexin, adiponectin, thyroxin and corticosterone, may also be affected by maternal nutritional state and will thus be considered in future studies.
In this study, males were not exposed to the HFD until the time they were paired to females. Thus, it is unlikely that this diet could affect their germ cells or male reproductive tract secretions. Prior studies have indicated that a paternal HFD can alter both of these factors and thereby affect offspring outcomes.Reference Binder, Hannan and Gardner 48 – Reference Binder, Beard and Kaitu’u-Lino 53 However, we cannot rule out the possibility that exposure to the HFD may compromise biparental care provided to the pups, as we observed in male and female California mice developmentally exposed to BPA or ethinyl estradiol.Reference Johnson, Javurek and Painter 117 To our knowledge, no studies to date have examined whether a HFD can alter maternal or paternal care. Thus, this possibility should be explored in future studies. Another likelihood that should be considered is that biparental care, which is unique to oldfield mice, other Peromyscus species and certain vole species, may mitigate some of the deleterious effects of a maternal HFD that are observed in laboratory mice, rats and other polygynous species, where the male reproductive partner generally does not show any significant parental care and may even cannibalize the pups if left in the cage with them. Our model may thus better approximate most human societies that are generally monogamous and where both parents share parental responsibilities.
In previous studies, we found that a maternal HFD with 60% calories as fat resulted in sex ratio distortions to males in parities two through four but not the first parity.Reference Rosenfeld, Grimm and Livingston 55 No such sex ratio bias was observed in the current studies that used a maternal HFD with 43.8%.
In conclusion, the current work with oldfield mice demonstrates that perinatal exposure to a HFD disrupts select behavioral domains and metabolic parameters. Puzzlingly, maternal HFD male offspring were more likely to solve the Barnes and reverse Barnes maze compared to control males. Female offspring exposed to a HFD exhibit increased anxiogenic behaviors. Voluntary physical activity in a home-cage setting was suppressed in HFD female and male offspring relative to controls. Consistently, HFD progeny also weighed more than controls. Further work is thus needed to determine the underpinning mechanisms of how a maternal HFD disturbs brain and metabolic programming in a potentially sex-specific manner.
Acknowledgments
The authors thank Dr Michael R. Felder at the University of South Carolina for providing the founder oldfield mice. The authors thank Dr Jonathan P. Thyfault for providing expertise in designing the indirect calorimetry experiments and Rebecca J. Welly for analyzing the indirect calorimetry data.
Financial Support
The studies were supported by NIH Grant 5R21ES023150 (to C.S.R.).
Conflicts of Interest
None.
Ethical Standards
The authors confirm that all procedures were performed in accordance with the recommendations detailed in the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health and have been approved by the University of Missouri Animal Care and Use Committee.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S2040174416000490










