Introduction
Perimenopausal disorders (PDs) result from variations in reproductive hormones levels or from gynecological lesions occurring during and after the menopausal transition period.Reference Santoro 1 – Reference Saso, Chatterjee and Georgiou 3 They typically present with a range of vasomotor, psychological, somatic and vaginal symptoms.Reference Santoro 1 , Reference Kuh, Wadsworth and Hardy 4 , Reference Mishra and Kuh 5 The International Classification of Diseases categorizes PDs into three main subtypes: menopausal and climacteric states (e.g. flushing, sleeplessness, headaches and poor concentration); perimenopausal bleeding (i.e. abnormal vaginal bleeding in peri- and postmenopausal period) and other PDs (e.g. atrophic vaginitis, atrophy of cervix or endometrium and other unspecified disorders). It is estimated that around 80% of postmenopausal women experience at least one symptom,Reference Woods and Mitchell 6 which can be bothersome that drives 10% of women to seek health care and potentially get a clinical diagnosed disorder.Reference Woods and Mitchell 6 These highly prevalent symptoms, and the corresponding disorders, can have a substantial negative impact on women’s general health and quality of life, with related costs to families, the health care system and society.Reference Utian 7 , Reference Avis, Colvin and Bromberger 8 Understanding the etiology of clinical diagnosed disorders, including their potential developmental origins, is one important step towards reducing the health burden.
In the past 30 years, it has become clear that characteristics such as birth weight and gestational age are not only key outcomes of infant health in their own right but also predict subsequent health and disease risks. For instance, low birth weight is associated with higher risks of coronary heart disease,Reference Barker 9 low adult bone massReference Buttazzoni, Rosengren and Tveit 10 and all-cause mortality,Reference Risnes, Vatten and Baker 11 whereas higher birth weight is associated with adult obesityReference Ahlgren, Wohlfahrt, Olsen, Sorensen and Melbye 13 and some site-specific cancers.Reference Johnsson, Haglund, Ahlsson and Gustafsson 12 , Reference Ahlgren, Wohlfahrt, Olsen, Sorensen and Melbye 13 Such findings support the hypothesis of the developmental origins of health and disease, which highlights the developmental plasticity of the human body,Reference Barker, Eriksson, Forsen and Osmond 14 and the consequent ways in which the prenatal environment exerts long-term influences on the development of metabolism and organ functions.Reference Barker 15 – Reference Yessoufou and Moutairou 17
It has been previously proposed that there may be developmental origins for ovarian ageing, according to reported associations between higher birth weight or extremes of birth weight and earlier menopause.Reference Treloar, Sadrzadeh, Do, Martin and Lambalk 18 , Reference Tom, Cooper and Kuh 19 Therefore, birth characteristics may plausibly also be linked to other menopause-related health markers such as symptoms during the perimenopausal period, but to our knowledge no earlier study has examined this. Instead, previously identified risk factors for perimenopausal symptoms are mainly restricted to those operating in early adulthood or during the menopausal transition period, such as lifestyle factors, life events and menopausal status.Reference Gold, Colvin and Avis 20 – Reference Hardy and Kuh 22 This lacking attention to potential early-life predictors likely reflects the fact that most studies rely on self-reported survey data in which information on early-life characteristics were poorly recorded, and few existing data resources enable following women prospectively from birth to menopause.
The current study addresses this research gap by using a well-established cohort, examining the developmental origins of three subtypes of diagnosed PDs recorded in the Swedish national patient registers, namely menopausal and climacteric states, perimenopausal bleeding and other PDs (e.g. atrophic vaginitis).
Methods
Study population
This study uses the Uppsala Birth Cohort Multigenerational Study (UBCoS Multigen). UBCoS Multigen is based on a well-defined original cohort of 14,192 live births in Uppsala University Hospital from 1915 to 1929.Reference Leon, Lithell and Vagero 23 For those cohort members still living in Uppsala as adults, we identified their offspring through the Multi-Generation Register and traced their birth data in archived obstetric records. Such identification and the linkage with other national registers can be done using the unique personal identification number in Sweden that each individual is assigned and remains unchanged throughout the lifetime. The starting point for the present analysis was the 4290 singleton female offspring who were born between 1936 and 1968, and were therefore aged 40–65 years during the follow-up period in 2001–2008. Then we excluded 258 females who died or emigrated before the start of follow-up, and a further 820 whose birth records could not be traced. The final study sample consisted of 3212 women. There was no evidence that the incidence of any of the three PDs subtypes differed significantly between women with traceable birth records and those without (all P>0.10).
Measures
The outcomes are based on clinical diagnosis of PDs identified in either inpatient or outpatient registers using the International Classification of Diseases, 10th Revision (ICD-10). The inpatient register includes public and private inpatient care in Sweden and reached complete nationwide coverage by 1987, while the outpatient register is available from 2001.Reference Ludvigsson, Andersson and Ekbom 24 Diagnoses classified were menopausal and climacteric states (code N95.1), perimenopausal bleeding (codes N92.4 and N95.0) and other PDs (codes N95.2, N95.8 and N95.9). These outcomes were studied separately due to potential differences in their etiology. In separate analyses for each subtype of PDs, we did not censor women who were diagnosed with a different subtype of PDs. Incidence of each subtype of PDs was identified as the first recorded diagnosis for the specific disorder during the follow-up period. Both main and contributory diagnoses were considered: the PD was listed as the main diagnosis in 87% of diagnoses (n=111) of menopausal and climacteric states, 91% of diagnoses (n=58) of perimenopausal bleeding and 71% of diagnoses (n=44) of other PDs. Among other PDs, the most common diagnosis was atrophic vaginitis [n=49 (79%)]. States associated with artificial menopause (ICD code N95.3) were not included among the studied outcomes.
Information on the primary exposure variables, birth weight in kilogram (kg) and gestational age were obtained from archived birth records. Gestational age was based on completed weeks from the last menstrual period of the mother. As an alternative exposure variable, ponderal index at birth was created as an indicator of the weight-for-length at birth [calculated as weight (kg)/birth lengthReference Saso, Chatterjee and Georgiou 3 (meters)]. Ponderal index measures the body mass of the infant and has been widely used in developmental origins studies.Reference Barker, Eriksson, Forsen and Osmond 14
Other early-life characteristics were included as potential confounding factors. Mother’s age, marital status (married or unmarried) and parity (1, 2, 3–9) at the birth of the index women (i.e. study population) were extracted from the archived birth records. Parental education was measured as the highest lifetime education of either parent from Census 1960, 1970 and 1990 or from the longitudinal database on education, income and employment and longitudinal integration database for health insurance and labor market studies (LISA) from 1985 onwards. We combined educational levels into four categories: elementary (elementary education <10 years), shorter secondary (senior high education <3 years), longer secondary (senior high 3+ years) and tertiary education.
Three adult characteristics of the index women were used to explore the social patterning of PDs and the role of socio-demographic factors in the associations between birth characteristics and the incidence of PDs. The woman’s lifetime number of live births was extracted from the Multi-Generation Register, and the woman’s own education and family disposable income (in fourths) in the year 2000 were obtained from the LISA register.
Statistical analysis
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs), with age used as the underlying timescale. Study subjects were followed from January 1, 2001 or their 40th birthday, whichever came later. Follow-up continued until December 31, 2008, their 65th birthday, death, emigration or the first diagnosis of the disorder in question, whichever happened earlier. The proportional hazards assumption was tested through the Schoenfeld residuals method, and there was no evidence of non-proportionality for any covariates (all P>0.11).
Models were fitted to predict each of our three outcome variables in turn. For each model, robust standard errors were applied to consider potential correlations between women with same biological mother. Birth weight and gestational age showed no evidence of nonlinearity as judged by including a quadratic term (all P>0.26), and were therefore entered as continuous variables. All models were first adjusted for year of birth (before 1944, 1945–49, 1950–54, 1955–1959, 1960 or later) of the index women to account for potential period effects. Birth weight was additionally adjusted for gestational age. Index women’s parental education, mother’s marital status, age and parity at birth were further added in the adjusted models. Finally, index women’s number of live births, education and family disposable income in year 2000 were included in the final models.
The proportion of missing data in study covariates was 0–5%. This was imputed using multiple imputation (25 imputations) under an assumption of missing at random. The imputation model included all other covariates, event indicators and the Nelson–Aalen estimator for cumulative hazard.Reference White and Royston 25 All Cox models were based on the imputed data.
Sensitivity analysis
To further assess the robustness of our results for women with natural menopausal transition, we restricted our sample to 2993 women who had never had a hysterectomy or oophorectomy before the start of the follow-up, defining these procedures using the Swedish Classification of Operations and Major Procedures (see Supplementary Table S1 for diagnostic codes).Reference Ingelsson, Lundholm, Johansson and Altman 26 In the sensitivity analyses, events of hysterectomy and oophorectomy were additional censored from follow-up – this was not done in the main analysis because the available operational codes are not sufficiently detailed to identify procedures that mean a woman has zero risk of disorders of natural menopause (e.g. bilateral removal of ovaries and uterus).
All analyses were conducted using STATA version 14.2 (StataCorp). All statistical tests were two-sided.
Results
During the 8 years of follow-up, 218 women out of 3212 had a diagnosed PD recorded in the patient registers (211 women in outpatient register and 7 women in inpatient register). Among them, 125 were diagnosed with menopausal and climacteric states, 61 with perimenopausal bleeding and 58 with symptoms linked to atrophic vaginitis or other unspecified PDs, and 26 women received more than one PD diagnosis.
As shown in Fig. 1, the incidence of menopausal and climacteric states increased steadily from age 48 to 65. For perimenopausal bleeding, most cases occurred before age 55, while other PDs were predominantly diagnosed after age 50. In general, the incidence rate of being diagnosed with any PDs peaked around age 50 (Supplementary Fig. S1).
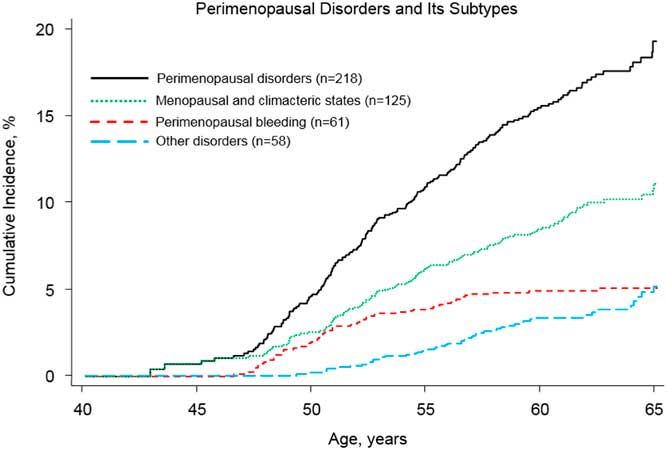
Fig. 1 Nelson–Aalen estimates of cumulative incidence curves for types of study outcomes: PDs and its three subtypes. The cumulative incidence was generated separately for each study outcome among 3212 women. For the three subtypes of PDs (i.e. menopausal and climacteric states, perimenopausal bleeding and other disorders), we did not censor women who were diagnosed with a different subtype of PDs. In total, 26 women received more than one subtype PDs diagnosis.
Table 1 shows study subjects’ early-life and adult characteristics, and corresponding PDs incidence rates. Rates of being diagnosed with PDs were generally higher among women with well-educated parents, and among women with a higher adulthood educational level themselves. Higher parity was associated with a higher rate of other PDs. Moreover, women with more number of live births during their adulthood had elevated rate of bleeding-specific diagnoses. There were no substantial differences in the incidence rate of PDs with other covariates. The above results were very similar after adjustment for birth year of the index woman (Supplementary Table S2).
Table 1 Descriptive statistics for the study covariates
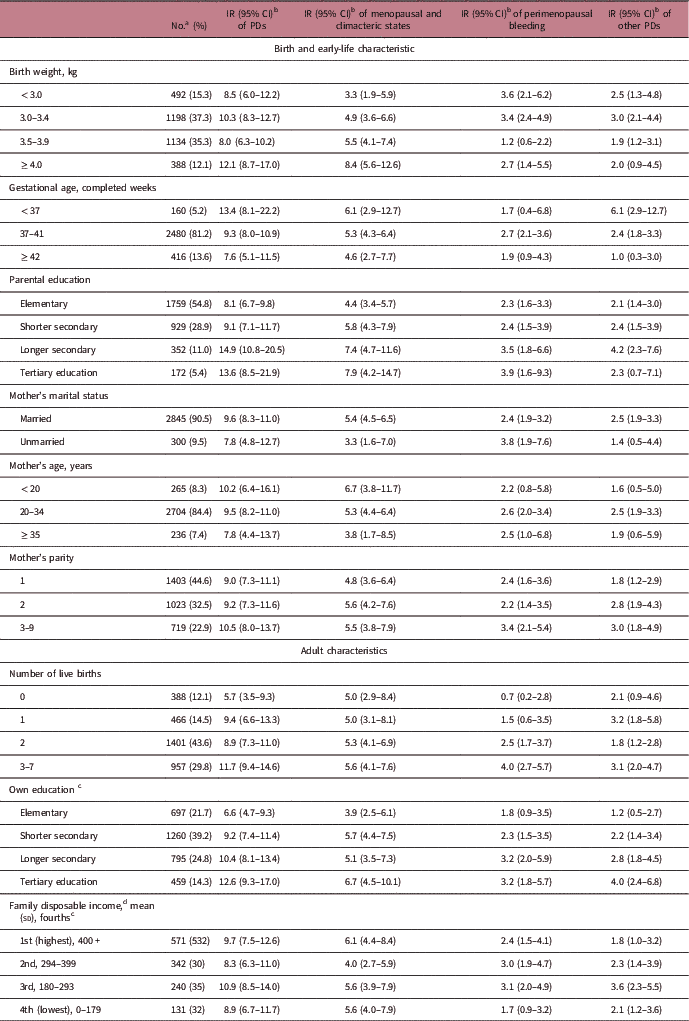
CI, confidence interval; IR, incidence rate; PDs, perimenopausal disorders.
a Numbers add up to <3212 for some covariates due to missing data.
b Per 1000 person-years.
c At year 2000.
d Presented in thousands Swedish Krona.
Menopausal and climacteric states
Table 2 presents the estimated HRs for three subtypes of PDs per unit increase in birth weight and gestational age. After minimally adjusting for the woman’s age, birth year and gestational age, increasing birth weight was linearly associated with higher rate of menopausal and climacteric states (HR=1.66 per 1 kg increase, 95% CI=1.14–2.41). This association was additionally confirmed by categorizing birth weight into four groups: <3.0, 3.0–3.4, 3.5–3.9 and ≥4.0 kg (see Supplementary Table S3 for alternative analyses with birth weight as a categorical variable). The strength of the association with size at birth was very similar when we instead used ponderal index as a relative measure of body mass (weight for length). Specifically, the HR was 1.28 (95% CI=1.07–1.54) for a 1 sd increase in birth weight, and 1.30 (95% CI=1.10–1.54) for a 1 sd in ponderal index (Supplementary Table S4). The magnitude of the association of birth weight with menopausal and climacteric states changed little after adjusting for other early-life and adult characteristics (Table 2 and Supplementary Table S5). There was no evidence of an association between gestational age and incidence of menopausal and climacteric states (Table 2).
Table 2 Associations of birth weight and gestational age with three subtypes PDs (n=3212)
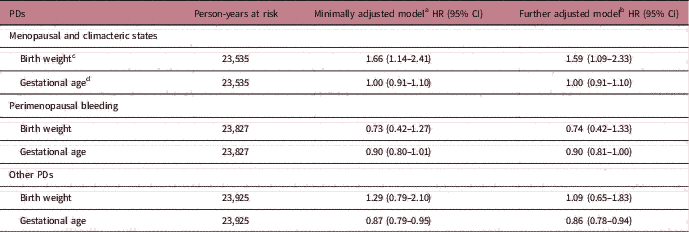
PDs, Perimenopausal disorders; HR, hazard ratio; CI, confidence interval.
a Minimally adjusted for birth year in categories, birth weight was additionally adjusted by gestational age.
b Further adjusted models additionally adjusted for parental education, mother’s marital status, age and parity at birth.
c Birth weight is change per 1 kg increase.
d Gestational age is change per additional completed week.
Perimenopausal bleeding disorders
For perimenopausal bleeding disorders, neither birth weight nor gestational age showed associations with incidence rates (all P>0.27, Table 2). These results did not change appreciably after adjustment for other early-life and adult socio-demographic characteristics (Table 2 and Supplementary Table S5).
Other PDs
Birth weight was not associated with incidence of other PDs. Rate of other disorders was, however, lower among women with longer gestational age (minimally adjusted HR=0.87, 95% CI=0.79–0.95 per completed gestational week, see Table 2). These results changed little after additionally adjustment for other early-life and adult characteristics (Table 2 and Supplementary Table S5).
Sensitivity analysis
All analyses were repeated with additional censoring for hysterectomy or oophorectomy. The results were very similar to those in the main analyses, except that the association of birth weight with menopausal and climacteric states was somewhat stronger (Supplementary Table S1).
Discussion
Using prospective data on 3212 Swedish women, this study investigated how birth weight and gestational age were associated with incidence of PDs. Higher birth weight was associated with increased incidence of menopausal and climacteric states such as flushing, sleeplessness or headache, whereas shorter gestational age was associated with increased incidence of other PDs (e.g. atrophic vaginitis). Neither birth weight nor gestational age showed associations with perimenopausal bleeding disorders. All results were very similar after adjustment for other early-life and adult socio-demographic characteristics.
Potential mechanisms
Compared to other important sources of health burden among women, such as reproductive cancersReference Yang, Reeves and Green 27 and early menopause,Reference Mishra 28 , Reference Hardy and Kuh 29 the developmental origins of PDs have not been adequately explored. Indeed, to our knowledge, this is the first study to investigate the association between birth characteristics and PDs. This limits direct comparisons of our research with other studies, but we can discuss our findings in respect of possible mechanisms based on previously identified adult risk factors.
Why higher birth weight is linked to the elevated risk of menopausal and climacteric states is unknown. One possible explanation is that high birth weight leads to adult overweight or obesity, which in turn increases the risk of menopausal and climacteric symptoms. The association between higher birth weight and adult higher body mass index (BMI) or obesity is fairly well established,Reference Johnsson, Haglund, Ahlsson and Gustafsson 12 although evidence regarding adult BMI and menopausal symptoms is more mixed. These mixed findings may reflect the effect modification by study subjects’ menopausal status with respect to the association between adult BMI and hormone/estrogen levels.Reference Gallicchio, Visvanathan and Miller 30 , Reference Freeman, Sammel, Lin and Gracia 31 One study has reported that overweight or obesity was associated with decreased risk of onset of menopausal symptoms but only among postmenopausal women, possibly reflecting the additional estrogen produced by their greater quantities of adipose tissue (higher estrogen levels can decrease the risk of menopause symptoms, in particular vasomotor-related symptomsReference Overlie, Moen, Holte and Finset 32 ).Reference Sabia, Fournier, Mesrine, Boutron-Ruault and Clavel-Chapelon 33 However, another study focused specifically on perimenopausal women (aged between 45 and 54) found that high BMI was associated with decreased estrogen levels and higher risk of menopausal symptoms.Reference Gallicchio, Visvanathan and Miller 30 This second study further reported a longer duration of perimenopause among obese women, suggesting that the decreased perimenopausal estrogen levels might reflect earlier onset of ovarian insufficiency (one phenomenon of early menopause).Reference Gallicchio, Visvanathan and Miller 30 This interpretation is supported by two epidemiological studies in which the association between higher birth weight and earlier menopause were foundReference Treloar, Sadrzadeh, Do, Martin and Lambalk 18 , Reference Tom, Cooper and Kuh 19 – although the degree of mediation through adult BMI on such association is unclear.
In summary, therefore, one possible explanation for the association between higher birth weight and the elevated risk of diagnosed menopausal states is that high birth weight predicts high adult BMI in the perimenopausal period. This is an area that requires further research, including consideration of potential upstream determinants (and potential targets for intervention) such as maternal obesity or diabetes. Regardless of the mechanism, our findings provide some supports for the developmental origins hypothesis that the perinatal period is a sensitive period for adult health, with exposure to adverse intrauterine environments potentially causing long-term health harms.Reference Barker 15
The lack of associations between birth characteristics and perimenopausal bleeding is arguably not surprising, given that abnormal bleeding is a marker for gynecological lesions or malignancies, which were not associated with birth weight in a recent meta-analysis.Reference Yang, Reeves and Green 27 The identified evidence of the association between shorter gestational age and increased risk of other PDs (e.g. vaginal or endometrial atrophy) could be the result of inadequate development of ovarian function and reproductive organs during prenatal period.
Strengths
The primary strength of the current research is its longitudinal design. This is uncommon in the field: most previous studies examining risk factors for menopause-related symptoms instead use cross-sectional data,Reference Pimenta, Leal, Maroco and Ramos 21 , Reference Pimenta, Leal, Maroco and Ramos 34 which limits their ability to explore potential long-term predictors such as birth characteristics. In this study, this association can be studied for the first time by linking archived birth records to national patient register. The prospective collection of early-life and premenopausal adult characteristics and the use of register-based diagnoses avoid potential problems of recall bias and differential misclassification of the outcomes.
Limitations and future directions
Nevertheless, our register-based design also arose some limitations of this study. Our outcomes were PDs diagnoses, as extracted from inpatient and outpatient registers. It is likely that only severe symptoms were captured in this study, because most women may not consider menopausal symptoms as disorders unless it seriously affects their general health. It can be assumed that we missed a considerable number of less severe cases among women who did not seek health care and who were treated in the primary care system. Differences in health-seeking behaviors might also explain why higher education was found to be associated with a higher risk of PDs in the present study, whereas previous survey-based research in United States reported the opposite association.Reference Gold, Colvin and Avis 20 As such, further research would be valuable to establish whether our findings can be generalized to all menopausal disorders.
Another limitation is that this study lacked the data to study surgical menopause, while additional censoring on hysterectomy and oophorectomy did not change the direction or magnitudes of the main results. Relatedly, although we categorized PDs in as much detail as the ICD allows, our outcomes were relatively broad in comparison to previous studies that have looked at symptom profiles (i.e. psychological, vasomotor, somatic and sexual discomfort profiles).Reference Mishra and Kuh 5 Thus, future studies with detailed outcome data would be valuable, to explore the early-life predictors of different aspects of PDs. A third limitation is that the lack of investigation on potential mediating pathways or accumulation of risk across the life course may underlie the observed associations (e.g. adult BMI or hormonal factors) to identify strategies for earlier interventions. Thus, further research into the mechanisms of the developmental origins of PDs is warranted. Additionally, the absence of information regarding women’s age at menopause limits our ability to detect the differences in timing of onset of PDs by birth characteristics. Finally, this study may not consider all potential maternal/family-level confounding factors, thus any causal interpretation needs to be made with caution. Although our sample size was not large enough to permit sibling analyses, such analyses could strengthen the case for causality would be one useful method to apply to larger data sets. Opportunities for doing this in Sweden will arise within the next 10 years, once women included in the Medical Birth Register (started in 1973) reach menopausal age.
Conclusion
This observational study provides, for the first time, suggestive evidence as to the developmental origins of PDs. We found that higher birth weight was associated with increased incidence of menopausal and climacteric states, and shorter gestational age was associated with increased incidence of other PDs such as atrophic vaginitis. No associations with birth characteristics were detected for perimenopausal bleeding. Future research should seek to replicate these findings in other populations in different contexts. If replicated, investigating the underlying causal mechanisms may shed further light on the etiology of this important class of disorders, and potentially identify targets for intervention.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S204017441800065X
Acknowledgments
None.
Financial Support
This work was supported by the European Union’s Horizon 2020 research and innovation programme [grant number 635316 (ATHLOS project)]; the Swedish Research Council (grant numbers 2013-5104, and 2013-5474); the Swedish Research Council for Health, Working Life and Welfare (grant numbers 2006-1518, 2013-1084 and 2013-1850) and the China Scholarship Council [grant number 201600160078 (M.G.)].
Conflict of Interest
None.
Ethical Standards
This study was approved by the Regional Ethics Committee in Stockholm, Sweden.





