Introduction
The effects of early-life environment on health in later life may differ for males and females. However, identifying general patterns of differences in susceptibility to specific insults in specific traits has proven difficult. Some authors have suggested that increased sensitivity to prenatal stress may be adaptive for females Reference Glover and Hill1 and found that the hypothalamic-pituitary-adrenal axis of females is more vulnerable to long-term programming. Reference Carpenter, Grecian and Reynolds2 However, many authors have suggested that males may have greater susceptibility to early-life effects Reference DiPietro and Voegtline3 because of a strategy to prioritize growth in the face of adversity, whereas females are more responsive to challenges. Reference Eriksson, Kajantie, Osmond, Thornburg and Barker4–Reference Sutherland and Brunwasser6
Males are at increased risk of preterm birth Reference Verburg, Tucker, Scheil, Erwich, Dekker and Roberts7,Reference Al-Qaraghouli and Fang8 and stillbirth. Reference Mondal, Galloway, Bailey and Mathews9 However, the sex ratio at conception is not different from 50:50, and sex bias in mortality varies throughout gestation, such that it is female-biased early in gestation and male-biased later, with total female prenatal mortality greater than total male mortality. Reference Orzack, Stubblefield and Akmaev10 Thus, if males adopt a riskier strategy (i.e., prioritizing growth), they only do so later in pregnancy. Sex differences in growth strategies and in the long-term effects of prenatal environment are likely influenced by the placenta. Reference Gabory, Roseboom, Moore, Moore and Junien11–Reference Clifton21 For example, it has been suggested that male placentas are more efficient but have less reserve capacity. Reference Eriksson, Kajantie, Osmond, Thornburg and Barker4,Reference Meakin, Cuffe, Darby, Morrison and Clifton22 However, direct tests of this hypothesis, or even a clear definition of “reserve capacity,” are lacking. Furthermore, sex differences in placental function show no clear patterns regarding prioritization of fetal growth or responsiveness to insults such as suboptimal maternal nutrition. Reference Christians23 Another limitation to understanding sex differences in fetal strategies is that many studies test the sexes separately, rather than explicitly testing the statistical interaction between sex and early-life environment, Reference DiPietro and Voegtline3,Reference Sutherland and Brunwasser6,Reference Chin and Christians24 even though the former approach is expected to generate spurious sex-specific effects. Reference Christians, Shergill and Albert25
The widespread use of inappropriate statistical approaches has the potential to obscure real patterns of sex-dependent effects. Moreover, the lack of a clear hypothesis will impede our understanding of sex differences in the effects of early-life environment. The purposes of the present study are to (1) define clear, testable predictions that follow from the hypothesis that males prioritize growth to an extent that makes them more susceptible to early-life adversity and (2) examine how analyzing the sexes separately may lead to spurious results. We do so using a large dataset that allows us to examine birthweights, placental weights as well as cognitive outcomes at 7 years of age in the same population, the National Collaborative Perinatal Project (NCCP).The NCCP collected data from ∼ 60,000 pregnancies, including information about pregnancy outcome, placental pathology, and follow-up psychological exams at various ages, Reference Hardy26 and so provides the opportunity to test hypotheses regarding sex differences in fetal strategies and the long-term effects of early-life environment. Although the NCCP began over 60 years ago, the data are of good quality Reference Hardy26,Reference Klebanoff27 and it forms the basis for many recent studies of placental pathology, Reference White, Grynspan, Van Mieghem and Connor28–Reference Ananth, Friedman, Lavery, VanderWeele, Keim and Williams31 cognitive development, Reference Gleason, Gilman and Sundaram32–Reference Lee, Papandonatos, Savitz, Heindel and Buka36 and other epidemiological questions. Reference Huang, Jiang, Xu, Lei and Zhang37–Reference Lei, Zhao and Huang42 Importantly for the present study, the biology underlying sex differences in fetal growth strategies would not have changed over this time period, and most of the variables used (e.g., birthweight, placental weight, and gestational age at birth) are straightforward to measure. While updated in some cases, many of the cognitive assessments are still in use.
Some early NCCP studies found associations between low birthweight and cognitive abilities at 7 years among term infants but did not examine the sex dependence of these effects. Reference Strauss and Dietz43–Reference Pylipow, Spector, Puumala, Boys, Cohen and Georgieff45 More recently, placental abruption and placental inflammation were found to be associated with impaired development at 4 years of age but not at 7 years, although again these studies did not examine sex dependence. Reference Ananth, Friedman, Lavery, VanderWeele, Keim and Williams31,Reference Chen, Lu, Xue, Ren, Zhang and Zhang33 Probable hypoxic-ischemic complication was also associated with intelligence quotient (IQ) at 7 years among term infants, but there was no interaction with sex. Reference Goldstein, Seidman and Buka46 When separating effects of acute and chronic hypoxia, indices of acute perinatal hypoxia were associated with a number of behavioral and cognitive outcomes at age 7 in both sexes, whereas chronic placental hypoxia (assessed by placental pathology) was associated with some traits in females but not males. Reference Anastario, Salafia, Fitzmaurice and Goldstein47 In contrast, the associations between prenatal bacterial infection and 7-year IQ, Reference Lee, Papandonatos, Savitz, Heindel and Buka36 and the incidence of psychosis Reference Lee, Cherkerzian and Seidman48 were stronger in males.
In the present study we use data from the NCCP to test predictions of the hypothesis that male fetuses prioritize growth, even in response to adversity, and that this strategy makes males more susceptible to mortality and long-term effects of prenatal insults. Specifically, we predict: (1) Among healthy pregnancies, males will be heavier than females and will have reduced placental reserve capacity, defined as lighter placentas for a given birthweight. (2) Among fetuses facing prenatal insults that ultimately lead to death, males will have maintained growth while females will have adjusted growth, resulting in a greater sex difference in birthweight among infants that die prenatally or shortly after birth. (3) As a result of taking a “riskier” strategy, males will be at greater risk of fetal or neonatal death. (4) Males will be more susceptible to long-term effects of early-life adversity such as low birthweight, prematurity, and maternal preeclampsia, a condition associated with impaired placental development. (5) Analyzing the sexes separately (rather than testing for the interaction between sex and adversity) will result in spurious sex-specific effects. Reference Christians, Shergill and Albert25
Method
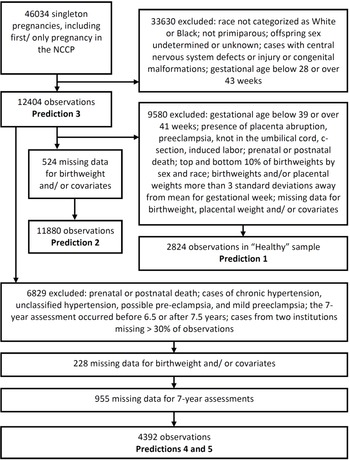
The NCPP has been described elsewhere, Reference Hardy26 and its data are publicly available (https://catalog.archives.gov/id/606622). Recruitment was carried out at 12 University hospitals in the United States (in Baltimore (Maryland), Boston (Massachusetts), Buffalo (New York), Memphis (Tennessee), Minneapolis (Minnesota), New Orleans (Louisiana), Philadelphia (Pennsylvania), Portland (Oregon), Providence (Rhode Island), and Richmond (Virginia), with two in New York (New York)). Maternal race was categorized as White or Black in over 90% of pregnancies, and so analyses were restricted to these two races. We used only singleton primiparous pregnancies Reference Reynolds, Roberts, Bodnar, Haggerty, Youk and Catov49 where offspring sex was assigned male or female. Where an individual had more than one pregnancy included in the NCCP, we included only the first study pregnancy. Fetal and neonatal deaths were included, but we excluded cases with central nervous system defects or injury or congenital malformations to exclude complications due to an intrinsic problem with the fetus. We excluded pregnancies where the gestational age was below 28 weeks (because fetal sex was missing for a large proportion of observations) or over 43 weeks; gestational age was based on the last menstrual period to the nearest week. We removed birthweights and placental weights that were more than 3 standard deviations away from the mean for their gestational week to objectively exclude biologically implausible values (Fig. 1).

Fig. 1. Selection of cohorts used to test each prediction.
For some analyses, we further restricted our sample to obtain a population of healthy pregnancies (Fig. 1). This was achieved by restricting to gestational ages of 39–41 weeks, inclusive, and removing cases of placenta abruption, preeclampsia, knot in the umbilical cord, c-section, induced labor, or where the child was known to have died either prenatally or postnatally at any age. We used a narrow range of gestational ages to avoid potentially confounding effects of placental weight relative to birthweight changing over the early-term period, Reference Ogawa, Matsuda, Nakai, Hayashi, Sato and Matsubara50 and because early-term births are associated with higher rates of adverse outcomes. Reference Sengupta, Carrion and Shelton51 C-sections were excluded because elective C-sections were very rare at the time of the NCCP. Reference Taffel, Placek and Liss52 In our healthy sample, we also removed the top and bottom 10% of birthweights by sex and race, as small-for-gestational age (SGA) and large for gestational age (LGA) are sometimes considered pregnancy complications.
Measures of early-life adversity
We defined SGA as birthweight below the 10th percentile, adjusting for gestational age, sex, and race. Prematurity was defined using World Health Organization categories, that is, very preterm (28–32 weeks, inclusive), moderate to late preterm (33–36 weeks, inclusive), and term (37–43 weeks, inclusive). Preeclampsia included severe preeclampsia and eclampsia, but excluded chronic hypertension, unclassified hypertension, possible preeclampsia, and mild preeclampsia. Mild preeclampsia was defined by the presence of one or more of the following: (1) systolic blood pressure of 140 mmHg or over, or rise of 30 mmHg above the usual level on at least two occasions, (2) diastolic blood pressure of 90 mmHg or over, or rise of 15 mmHg above usual on at least two occasions, (3) proteinuria of “significant degree” (+1 or more/>30 mg) on two successive days, (4) persistent edema of hands and face. Severe preeclampsia was defined by the presence of one or more of the following: (1) systolic blood pressure of 160 mmHg or over on at least two occasions, (2) diastolic blood pressure of 110 mmHg or over on at least two occasions, (3) proteinuria of 5 g or more (+3/+4), (4) oliguria (400 cc or less in 24 h), (5) cerebral or visual disturbances, retinopathy, headache, associated epigastric pain, (6) pulmonary edema or cyanosis. Eclampsia was defined as preeclampsia associated with convulsion and/or coma.
Assessments at age 7
We examined assessments made on continuous scales at 7 years of age, including seven subtests of the Wechsler Intelligence Scale for Children (WISC), three tests from the Wide Range Achievement Test (WRAT), the Bender Gestalt Test for Young Children and the auditory-vocal association test from the Illinois Test of Psycholinguistic Abilities. The subsets of the WISC included verbal tests (vocabulary, information, comprehension and digit span, and a measure of verbal memory) and performance tests (picture arrangement, block design, and coding), with scores on each subset ranging from 0 to 20. Verbal tests were used to derive a verbal IQ score, while the performance tests were used to derive a performance IQ score, and these two IQ scores were in turn used to derive the full-scale IQ; IQ scores ranged from ranged from 44 to 156. The WRAT tests included reading, spelling, and arithmetic components and scores ranged from 0 to 97. The auditory-vocal tests assessed the ability to relate verbal symbols and provided both a standard score (ranging from −300 to 227) and an estimate of language age (ranging from 0 to 900). For all of these assessments, a higher score indicated improved performance, except for the Bender Gestalt, where the score was a measure of the number of errors made in drawings (ranging from 0 to 21). These tests are described in further detail elsewhere. Reference Lee, Papandonatos, Savitz, Heindel and Buka36,Reference Seidman, Buka, Goldstein, Horton, Rieder and Tsuang44
Pregnancies were excluded where the 7-year assessment occurred before 6.5 years of age or after 7.5 years. To reduce the potential effects of bias, we excluded data from two institutions (Children’s Hospital Medical Center, Boston and Metropolitan Hospital, New York) where more than 30% of observations (including only livebirths) were missing one or more of the 7-year assessments (Fig. 1).
Covariates
Covariates included maternal age, race, education (number of years), socioeconomic status (SES), and smoking status (yes/no). SES was categorized as bottom third or above.
Statistical Analyses
We used SAS (Version 9.4) for all analyses, including linear models (GLM procedure), nonparametric comparison of means (NPAR1WAY procedure), and survival analysis (LIFETEST procedure). Models are described in further detail below.
Results
Prediction 1: Among healthy pregnancies, males will be heavier than females and will have reduced placental reserve capacity
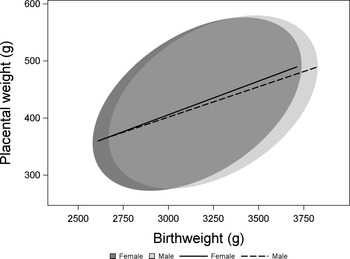
2824 observations met our criteria of healthy pregnancies and had no missing values for birthweight, placental weight, sex or any covariate (Fig. 1). Samples with data missing for one or more variable did not differ from those with complete data for all of the variables included in this analysis (Supplementary Table S1). While both birthweight and placental weight deviated significantly from a normal distribution (Kolmogorov–Smirnov test P < 0.01 in both cases), values of skewness, and kurtosis were low (birthweight skewness: 0.05; birthweight kurtosis: −0.7; placental weight skewness: 0.36, placental weight kurtosis: 0.13). We analyzed the effect of sex on birthweight using a model including effects of gestational age, maternal race, SES, and smoking status. Male newborns were heavier than female newborns (male: 3214 ± 7 g, female: 3123 ± 7 g, P < 0.0001). A similar model found no difference in placental weight between the sexes (male: 426 ± 2 g, female: 421 ± 2 g, P = 0.14). To test whether there was a sex difference in the weight of the placenta relative to birthweight, we included birthweight and the interaction between birthweight and sex as additional terms in the model to avoid problems associated with the use of ratios. Reference Christians, Grynspan, Greenwood and Dilworth53 Adjusting for birthweight, there was no difference in placental weight between the sexes (male: 425 ± 2 g, female: 432 ± 2 g, P = 0.29; Fig. 2).

Fig. 2. The relationship between birthweight and placental weight among and females from healthy pregnancies. Data are presented as 90% confidence ellipses (ELLIPSE statement in proc SGPLOT, SAS, Version 9.4) with least-squares regression lines.
Prediction 2: There will be a greater sex difference in birthweight among infants that die prenatally or shortly after birth
If there are sex differences in growth strategy, whereby females are more responsive to adversity while males prioritize growth, we predict that the difference in birthweight between males and females will be greater among infants that die prenatally or shortly after birth (within 27 days) than among those who survive. Among fetuses that do not survive, females are expected to have reduced their growth, whereas males are expected to have attempted to maintain their growth, even in unfavorable conditions. While we did not examine specific causes of death, at least some cases would be due to chronic conditions in response to which fetuses might have adjusted growth. We included early postnatal death because this may have been due to a prenatal complication.
When not restricting to healthy pregnancies, there were 11,880 pregnancies with complete data (Fig. 1). Because this analysis included a wider range of gestational age (28–43 weeks, inclusive), we log-transformed birthweight prior to analysis to improve the fit of the model. To assess whether the sex difference in birthweight among infants that die was greater than among infants who survived, we tested for an interaction between sex and survival. The model included effects of sex, survival, the interaction between sex and survival, gestational age, the interaction between gestational age and sex, the three-way interaction between sex, survival, and gestational age, as well as maternal race, SES, and smoking status. The three-way interaction between sex, survival, and gestational age was highly significant (P < 0.0001), indicating that the interaction of interest, that is, between sex and survival, varied with gestational age. To understand this interaction, we repeated the analysis separately for prematurity categories, that is, very preterm (28–32 weeks), moderately preterm (33–36 weeks), and term (37–43 weeks), removing gestational age from the model. The interaction between sex and survival on birthweight was only significant among very preterm births (P = 0.0002), with the difference in birthweight between males and females greater among those who did not survive, as predicted (Fig. 3). The interaction was not significant among moderately preterm and term births (P = 0.06 and P = 0.66, respectively, Fig. 3), that is, our prediction was not supported for these gestational age categories.

Fig. 3. Birthweight in infants that died prenatally or within 27 days of birth and in those that survived. Values are least squares means ± standard error from linear models including effects of sex, survival, the interaction between sex and survival, race, SES, smoking status, and BMI, performed separately for each gestational age category. Birthweight was log-transformed prior to analysis and back-transformed for presentation. * indicates a significant (P < 0.01) interaction between sex and survival within a gestational age category, whereby the difference in birthweight between males and females is greater among those who did not survive.
Prediction 3: Males are at greater risk of fetal or neonatal death
When not restricting to pregnancies with data for birthweight, placental weight, and covariates, there were 12,404 pregnancies (Fig. 1). Males were born slightly but significantly earlier (male mean: 39.1 weeks, female mean: 39.3 weeks) whether tested with a nonparametric Kruskal–Wallis test (χ1 = 12.23, P = 0.0005) or with survival analysis (Wilcoxon χ1 = 12.23 P = 0.0005). However, males were not more likely to die prenatally or prior to 28 days postpartum or following delivery (Table 1). Survival analysis yielded similar results. Stratifying by preterm status yielded similar results, although there was a nonsignificant trend for increased mortality of males among term births (Table 1).
Table 1. Numbers of infants who died prenatally or prior to 28 days

Included and excluded observations are as described in Fig. 1 for prediction 3 (N = 12,404).
Prediction 4: Males will be more susceptible to long-term effects of early-life adversity
We tested whether the effects of early-life adversity (low birthweight, prematurity, and preeclampsia) on cognitive outcomes at age 7 were more severe in males. After restricting to livebirths, excluding known postnatal deaths, excluding observations where the 7-year assessment occurred before 6.5 years of age or after 7.5 years, and excluding data from two institutions with high rates of missing values (>30%), there were 7210 pregnancies with complete data for all variables apart from the 7-year assessments. The proportion of pregnancies that were missing assessments at 7 years did not differ by sex, SGA status, prematurity status, or preeclampsia status, although there were non-significant trends for a higher proportion of missing values in infants with SGA, and a lower proportion of missing values in preeclamptic pregnancies (Table 2).
Table 2. Numbers of children missing 7-year assessments

The number of observations is lower for preeclampsia status because we excluded 1863 cases with chronic hypertension, unclassified hypertension, possible preeclampsia, or mild preeclampsia.
We analyzed all 7-year assessments using linear models including effects of SGA, prematurity, preeclampsia, sex, pairwise interactions between sex and each of SGA, prematurity, preeclampsia, as well as maternal race, age, education, SES, and smoking status. Children born SGA had significantly reduced performance at 13 out of 16 of the tests; only the Bender Gestalt Test and the block design and vocabulary subsets of the WISC were not affected by SGA status (Table 3). Very preterm birth was associated with significantly reduced performance at 13 out of 16 tests; only the coding, comprehension, and information subsets of the WISC were not affected (Table 4). There were no differences between children born moderately preterm and at term (Table 4). Severe preeclampsia was associated with reduced performance in only three tests: the Bender Gestalt Test, performance IQ and the picture arrangement subset of the WISC (Table 5).
Table 3. Effects of being born small-for-gestational age (SGA) on cognitive assessments at 7 years of age

Values are least squares means ± standard error from linear models including effects of SGA, prematurity, preeclampsia, sex, pairwise interactions between sex and each of SGA, prematurity, preeclampsia, as well as maternal race, age, education, SES, and smoking status. Analyses were repeated separately for each sex, and P-values for the effect of SGA are provided. We deemed a sex-specific effect to be spurious if the sex by SGA interaction was not significant, but there was a significant effect of SGA in one sex but not the other when analyzing the sexes separately.
Table 4. Effects of being born premature (PT) on cognitive assessments at 7 years of age

VPT = very preterm (28–32 weeks); MPT = moderately preterm (33–36 weeks, inclusive); values are least squares means ± standard error from linear models including effects of SGA, prematurity, preeclampsia, sex, pairwise interactions between sex and each of SGA, prematurity, preeclampsia, as well as maternal race, age, education, SES, and smoking status. Differences were assessed using Tukey’s multiple comparisons. Analyses were repeated separately for each sex, and P-values for the effect of prematurity are provided. We deemed a sex-specific effect to be spurious if the sex by prematurity interaction was not significant, but there was a significant effect of prematurity in one sex but not the other when analyzing the sexes separately.
Table 5. Effects of preeclampsia (PE) on cognitive assessments at 7 years of age

Values are least squares means ± standard error from linear models including effects of SGA, prematurity, preeclampsia, sex, pairwise interactions between sex and each of SGA, prematurity, preeclampsia, as well as maternal race, age, education, SES, and smoking status. Analyses were repeated separately for each sex, and P-values for the effect of preeclampsia are provided. We deemed a sex-specific effect to be spurious if the sex by preeclampsia interaction was not significant, but there was a significant effect of preeclampsia in one sex but not the other when analyzing the sexes separately.
Although SGA, prematurity, and preeclampsia had significant effects on many traits (Tables 3–5), only one interaction with sex was significant. SGA was associated with a reduction in performance at the vocabulary subset of the WISC in girls but not boys (Table 3). However, given that we tested three interactions in each of 16 traits, that is, 48 tests, we expected approximately two tests to be significant due to chance given a Type I error rate of 0.05. Thus, we found little evidence of sex-dependent effects. Considering sex effects, rather than sex by adversity interactions, girls had significantly higher performance in the spelling and reading WRAT, and the coding, comprehension, digit span and vocabulary subsets of the WISC (Table 6).
Table 6. Effects of sex on cognitive assessments at 7 years of age

Values are least squares means ± standard error from linear models including effects of SGA, prematurity, preeclampsia, sex, pairwise interactions between sex and each of SGA, prematurity, preeclampsia, as well as maternal race, age, education, SES, and smoking status.
Prediction 5: Analyzing the sexes separately will result in spurious sex-specific effects
Many studies test for sex-dependent effects by analyzing the sexes separately. We repeated the analyses above separately for each sex to assess whether this approach resulted in spurious sex-dependent effects compared with analyses that combined the sexes and tested for a sex by adversity interaction. For SGA, 13 traits out of 16 showed spurious sex-specific effects, that is, where the effect of SGA was significant in one sex but not the other, even though the sex by SGA interaction was not significant in the combined analysis (Table 3). For prematurity and for preeclampsia, 12 and 2 out of 16 traits showed spurious sex-specific effects, respectively (Tables 4 and 5).
Discussion
Prediction 1: Among healthy pregnancies, males will be heavier than females and will have reduced placental reserve capacity
Males were heavier at birth, consistent with findings that males are heavier as early as the first trimester. Reference Bukowski, Smith and Malone54–Reference Kiserud, Piaggio and Carroli56 We found no difference in placental weight between males and females, in contrast to some studies that have found males to have larger placentas. Reference Roseboom, Painter and De Rooij57–Reference Muralimanoharan, Gao, Weintraub, Myatt and Maloyan61 Placental weight, adjusting for birthweight by including it as a covariate, did not differ between males and females. Thus, we found no evidence that male placentas are more efficient or maintain a reduced reserve capacity, defined as lighter placentas for a given birthweight. However, it remains possible that differences in placental structure and function could lead to sex differences in the ability of the placenta to respond to adverse conditions such as reduced nutrient supply. Reference Christians23,Reference Salazar-Petres, Carvalho, Lopez-Tello and Sferruzzi-Perri62
Prediction 2: There will be a greater sex difference in birthweight among infants that die prenatally or shortly after birth
If females are more responsive to prenatal insults, while males prioritize growth, we expected that female fetuses that died prenatally or shortly after birth would have reduced their growth more than males in an attempt to survive. As a result, the difference in birthweight between females that survived and those that did not would be greater than that among males, leading to a significant sex by survival interaction. This prediction was supported, but only among those born very preterm. While this result might reflect a difference in strategy between the sexes, an alternative explanation is that males and females may have been susceptible to different causes of death, whereby females were more likely to die from chronic impairments and conditions that reduced growth over a longer time period. We were not able to distinguish these alternatives with the available data. However, given that the risks of gestational diabetes, preterm birth, placental abruption and stillbirth are higher in male pregnancies, Reference Verburg, Tucker, Scheil, Erwich, Dekker and Roberts7–Reference Mondal, Galloway, Bailey and Mathews9,Reference Challis, Newnham, Petraglia, Yeganegi and Bocking63,Reference Broere-Brown, Adank and Benschop64 while the risks of preterm preeclampsia may be higher in female pregnancies, Reference Verburg, Tucker, Scheil, Erwich, Dekker and Roberts7,Reference Broere-Brown, Adank and Benschop64 it is plausible that the prevalence of specific types of insults differs between the sexes.
A similar examination of sex-dependent responses to preeclampsia found a greater reduction in fetal growth in males than in females, Reference Reynolds, Roberts, Bodnar, Haggerty, Youk and Catov49 a result opposite to our prediction. In addition to changes in fetal growth, another potential response to prenatal insult such as maternal vascular malperfusion is accelerated placental maturation, a compensatory response to improve placental transport capacity. However, in this population we previously found that the odds of accelerated placental maturation do not differ by sex. Reference Christians and Grynspan65
Interpreting the results of such human studies is difficult due to the presence of potentially confounding effects, for example, if males and females are subject to different insults. Animal models, allowing manipulation of prenatal conditions, are useful in this respect. In rodents, the fetal growth response to a reduction in food part way through pregnancy did not differ between the sexes, again inconsistent with the view that males prioritize growth more, or have less placental reserve capacity, than females. Reference Christians, Shergill and Albert25 Similarly, surgical models of reduced uterine perfusion pressure reduce fetal weight in both sexes. Reference Siragher and Sferruzzi-Perri66 While maternal inhalation hypoxia sometimes has sex-dependent effects of fetal growth, results from different studies are conflicting. Reference Siragher and Sferruzzi-Perri66
Prediction 3: Males are at greater risk of fetal or neonatal death
While we did not observe a difference in perinatal mortality between males and females, a meta-analysis of over 30 million births found an increased risk of stillbirth among males. Reference Mondal, Galloway, Bailey and Mathews9 However, this discrepancy is likely due to the lower sample size in the present study; the increased risk estimated in the meta-analysis (∼10%) was the same as we observed. The mechanisms underlying sex differences in the risk of fetal and neonatal death are not known. Some have speculated that higher mortality in males is the result of increased growth rates making fetuses vulnerable to stressors. Reference Eriksson, Kajantie, Osmond, Thornburg and Barker4–Reference Sutherland and Brunwasser6,Reference Mondal, Galloway, Bailey and Mathews9 However, such interpretations focus on deaths in mid- to late-gestation, ignoring the female-biased mortality that occurs early in gestation, when fetal loss may not be recognized, or fetal sex not recorded. Reference Orzack, Stubblefield and Akmaev10 Thus, it may be that compromised males are more likely to survive past early gestation than compromised females, such that male deaths are more likely to be observed, rather than more likely to occur.
Prediction 4: Males will be more susceptible to long-term effects of early-life adversity
We analyzed 7-year assessments using models that included effects of SGA, prematurity and preeclampsia simultaneously. We tested three interactions (between sex and each of SGA, prematurity and preeclampsia) in each of 16 traits, that is, 48 tests, and found only one interaction to be significant. For comparison, we expected approximately 2 out of 48 tests to be significant due to chance given a Type I error rate of 0.05. The one interaction that was significant suggested that girls were more affected by SGA than boys.
Although we found few interactions with sex, the main effects of SGA and prematurity were significant for most of the 7-year traits we examined, with infants born SGA and/or very premature having lower performance, consistent with previous studies of low birthweight and intrauterine growth restriction in this population. Reference Strauss and Dietz43–Reference Pylipow, Spector, Puumala, Boys, Cohen and Georgieff45 In this population, IQ at 7 years was also associated with birthweight within the normal range, although this association was stronger in males when analyzing same-sex siblings. Reference Matte, Bresnahan, Begg and Susser67 In other populations, extremely low birthweight has been associated with reduced cognitive and academic achievement in both sexes, Reference Grunau, Whitfield and Fay68 although cognitive performance was more impacted by extreme prematurity in males. Reference Marlow, Wolke, Bracewell and Samara69 The mechanisms underlying the effects of SGA and prematurity on long-term cognitive outcomes remain active areas of research. A compromised in utero environment could directly affect brain structure and/or increase fetal exposure to glucocorticoid levels as a result of reduced placental 11β-HSD-2 expression, and/or alter fetal exposure to serotonin produced by the placenta. Reference O’Donnell and Meaney70,Reference Brummelte, Mc Glanaghy, Bonnin and Oberlander71 . In contrast to SGA and prematurity, severe preeclampsia was associated with reduced performance in only three tests. Previous work in this population also found a stronger effect of low birthweight than of hypoxia and ischemia, which was defined in part by the presence of preeclampsia. Reference Seidman, Buka, Goldstein, Horton, Rieder and Tsuang44 Other work did find an association between probable hypoxic-ischemic complication and reduced full-scale IQ at 7 years, and no interaction with sex, Reference Goldstein, Seidman and Buka46 whereas in the present study the effect of severe preeclampsia on full-scale IQ was not significant, although performance IQ was reduced. Placental pathology features characteristic of chronic placental hypoxia were associated with reduced verbal IQ in girls only. Reference Anastario, Salafia, Fitzmaurice and Goldstein47
A negative effect of preeclampsia on intellectual abilities has also been observed in other populations, Reference Pinheiro, Brunetto, Ramos, Bernardi and Goldani72–Reference Morsing and Maršál74 although reductions in intellectual performance were minimal or not significant at older ages. Reference Seidman, Laor, Gale, Stevenson, Mashiach and Danon75–Reference Factor-Litvak, Straka and Cherkerzian79 Few of these studies report sex-dependent effects, although gross-motor scores were more affected in girls, while fine-motor scores were more affected in boys. Reference Ounsted, Moar, Good and Redman80
Prediction 5: Analyzing the sexes separately will result in spurious sex-specific effects
Analyzing the sexes separately frequently resulted in significant effects of SGA and/or prematurity in one sex but not the other, even though the sex by adversity interaction was not significant when the sexes were analyzed together. We defined these situations as spurious sex-specific effects, and these were observed in 13 out of 16 traits for SGA, 12 out of 16 traits for prematurity, and 2 out of 16 traits for preeclampsia. While the 16 traits we studied are not independent, these results illustrate that analyzing the sexes separately will frequently produce misleading sex-specific effects.
The problem with analyzing the sexes separately is illustrated by the following scenario: if the difference between SGA and non-SGA infants is significantly different from zero in males, but not in females, it does not necessarily follow that the effect size in males is significantly different from the effect size in females; the non-significant effect in females is not evidence that the effect size in females is actually zero. The false sex-specific effects that are observed when the sexes are analyzed separately reflect an inflation of the statistical error rate. When the sexes are analyzed together and the sex by adversity interaction is explicitly tested, there is a single opportunity to commit a type I or type II error. However, when the sexes are analyzed separately, there are two opportunities to commit each type of error. Even if sample sizes are large, the probability of a type II error (accepting the null hypothesis when it is false) may be substantial for a small effect size. As a result of type II errors, there may be a substantial probability of observing a significant effect in one sex but not the other, even if the actual effect size is the same in both sexes.
While a diversity of statistical approaches are used in this field, many studies test the sexes separately, Reference DiPietro and Voegtline3,Reference Sutherland and Brunwasser6 rather than testing interactions. We suggest that some of the reported sex-specific effects may therefore be false positives. Such false positives will obscure real patterns of sex-dependent effects.
Limitations
We restricted our analyses to primiparous White and Black participants to obtain a more homogenous sample, and to avoid potentially confounding effects. However, as a consequence, our results may not generalize to other groups. Similarly, we included only pregnancies ending at 28 weeks of gestation or later, and thus would not have observed differences occurring earlier.
Our definitions of early-life adversity were limited by the availability of data. For instance, SGA was based entirely on birthweight, and thus would have included fetuses that were intrinsically small but healthy; improved definitions of fetal growth restriction involve both estimates of fetal weight and Doppler abnormalities. Reference Melamed, Baschat and Yinon81 Furthermore, the definition of severe preeclampsia has changed since the NCCP study. Briefly, in NCCP data, severe preeclampsia was defined as the presence of one or more features, whereas the current definition includes severe hypertension and one or more features. 82 Furthermore, even using the current definition, preeclampsia is heterogeneous, Reference Leavey, Benton, Grynspan, Kingdom, Bainbridge and Cox83 which could explain why fewer long-term effects were observed for this condition. Although we did not detect any patterns to missing data, these could have introduced bias, for example, if more severe complications were more likely to be missing data because of urgency during delivery. Potentially, these limitations could have led to the inclusion or exclusion of a greater number of complications for one sex than the other.
Conclusions
Among very preterm infants, the difference in birthweight between females that survived and those that did not was greater than that among males. This result is consistent with the hypothesis that females are more responsive to prenatal insults (and so reduced their growth in an attempt to survive), while males prioritize growth. However, we did not observe this pattern at later gestational ages. Infants born SGA and/or very premature had lower performance in most of 7-year assessments, but there was only one significant interaction between sex and adversity, which suggested that girls were more affected by SGA than boys. Analyzing the sexes separately, rather than testing the adversity by sex interaction, resulted in numerous spurious sex-specific effects. The hypothesis that male fetuses prioritize growth is very popular Reference Eriksson, Kajantie, Osmond, Thornburg and Barker4,Reference Meakin, Cuffe, Darby, Morrison and Clifton22 despite little direct support. Overall, we found little support for our predictions deriving from this hypothesis. In light of our results, and the excess female mortality early in gestation, Reference Orzack, Stubblefield and Akmaev10 the view that males have increased vulnerability as a result of prioritizing growth and reduced placental reserve should be reevaluated.
Given the potential for spurious effects illustrated by the present study, tests for sex-dependent effects must use robust statistical approaches, for example, interaction terms, effect modification, Reference Vanderweele84 Bayesian approaches, Reference Chin and Christians24 or other explicit tests of whether effects differ between the sexes. Studies that simply analyze the sexes separately should be viewed, at best, with caution. A better understanding of sex-dependent fetal growth strategies and susceptibility to prenatal adversity will identify whether there is a need for sex-dependent interventions for specific pregnancy complications and, if so, how such interventions might be developed.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S2040174422000204
Acknowledgements
Bernard Crespi and Pablo Nepomnaschy provided helpful discussion at early stages of this study, and two anonymous reviewers provided detailed and constructive comments.
Financial support
This study was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (JKC; grant number RGPIN-2016-04047).
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans) and with the Helsinki Declaration of 1975, as revised in 2008. This study was based on publicly available, anonymized data and therefore did not require approval by an institutional committee.