Published online by Cambridge University Press: 01 June 2009
1. The relationship between vapour/liquid equilibrium coefficient for a tainting substance in cream (mc) as compared with the coefficient for the substance in skim-milk (ms) at 100° C. is given by the formula
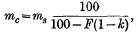
where F is the percentage fat content of the cream, and k is the butterfat/skim-milk distribution coefficient at 100° C.
2. The values for the vapour/liquid equilibrium coefficients for acetoin, diacetyl, acetylpropionyl, acetyl-iso-butyryl, acetylvaleryl, acetylcaproyl and acetylbenzoyl in cream, computed by the formula from the equilibrium coefficients for the substances in 4·5% lactose solutions and the distribution ratios at 100° C, were in good agreement with the experimental results for these substances in cream.
3. It was shown that high solubility of a substance in butterfat relative to solubility in skim-milk greatly reduces the steam volatility of the substance from cream.
4. It was concluded that steam volatility of a substance from cream may be affected by temperature of deodorization because of the effect of temperature on the butterfat'skim-milk distribution coefficient.