Recently, milk consumption has been predicted to increase globally with the increasing world population, with special interests in goat milk compared with cow milk due to greater contents of short- and medium-chain fatty acids (FA), as well as small-size fat globules (Chilliard et al. Reference Chilliard, Rouel, Ferlay, Bernard, Gaborit, Raynal-Ljutovac, Lauret, Leroux, Williams and Buttriss2006). Milk yield, composition, and FA profile can be altered by modifying the feeding regimen and phytogenic supplementation (Kholif et al. Reference Kholif, Gouda, Morsy, Salem, Lopez and Kholif2015, Reference Kholif, Morsy, Gouda, Anele and Galyean2016, Reference Kholif, Matloup, Morsy, Abdo, Abu Elella, Anele and Swanson2017a).
Plant parts such as leaves and seeds contain bioactive compounds like essential oils, saponins, and tannins (Cedillo et al. Reference Cedillo, Kholif, Salem, Elghandour, Vázquez, Alonso, Barbabosa, Chagoyán and Reyna2015; Kholif et al. Reference Kholif, Matloup, Morsy, Abdo, Abu Elella, Anele and Swanson2017a; Matloup et al. Reference Matloup, Abd El Tawab, Hassan, Hadhoud, Khattab, Khalel, Sallam and Kholif2017) with some antimicrobial and anthelmintic properties, which can be utilised in ruminants to improve feed utilisation, animal performance, and milk nutritive value (Kholif et al. Reference Kholif, Gouda, Morsy, Salem, Lopez and Kholif2015, Reference Kholif, Morsy, Gouda, Anele and Galyean2016). Extracts and whole plants containing these bioactive compounds can provide low-cost alternative for improving feed efficiency and milk production (Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013; Salem et al. Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014).
Mustard (Brassica juncea) is an oil seed crop, mainly grown as a condiment, with many advantages like drought and disease resistance. Mustard meal is an excellent protein source in ruminant nutrition due to its crude protein (CP) content and well-balanced amino acid composition (Khandaker et al. Reference Khandaker, Uddin, Sultana and Peters2012). However, mustard inclusion in ruminant feeding is limited because of elevated glucosinolates. Reductions in feed intake, due to low acceptability, have been reported in dairy cows when mustard seeds or meal were fed at high levels (i.e. 150 g/kg dry matter (DM) of concentrate mixtures) (Moss, Reference Moss1975). To the best of our knowledge, little research has been conducted to evaluate the feeding value of mustard seeds for ruminants.
Cumin (Cuminum cyminum) is a flowering plant in the family Apiaceae, and several in vitro cell culture studies showed that cumin extract has some antioxidant compounds, antibacterial activity, and can also activate digestive enzymes (Tajkarimi et al. Reference Tajkarimi, Ibrahim and Cliver2010). In vitro experiments showed increased DM and organic matter (OM) digestibility in forages with a reduction in methane emission with the addition of cumin seed powder (Chaudhry & Khan, Reference Chaudhry and Khan2012). Cumin seed extract resulted in increased milk production in lactating goats when supplemented at 1·27% DM intake (Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013). Inclusion of cumin and mustard seeds enhanced the nutritive value of milk by lowering saturated fatty acids (SFA) and increasing conjugated linoleic acid (CLA) and unsaturated fatty acids (UFA) concentration (Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013), which are very beneficial in human nutrition.
It was hypothesised that the bioactive compounds in mustard and cumin seeds will enhance ruminal fermentation and feed utilisation, resulting in improved milk production. It was also hypothesised that goats can handle the bitter taste of the secondary metabolites in cumin and mustard seeds. Therefore, concentration of chemical constituents of mustard and cumin and the effect of whole mustard and cumin seeds inclusion at 10 g/d in the diets of Damascus goats on feed utilisation, milk production and milk nutritive value (SFA, UFA and CLA contents) were studied for 12 weeks.
Materials and methods
Goats were managed in accordance with the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, Champaign, IL). The trial was conducted at the experimental farm of Animal Production Research Institute (Egypt) and at the Laboratory of Dairy Animal Production, National Research Centre (Egypt).
Goats, feeding and experimental design
Fifteen multiparous lactating Damascus goats (2 ± 1 parity) at first week of lactation (6 ± 2 d of lactation), with about 950 g milk/d on average (44·2 ± 0·5 kg body weight) were randomly assigned to three experimental groups (5 goats per treatment) for 12 weeks. Goats were balanced to treatment for expected average milk yield. Kids were kept with their mother all through the experimental period with exception of days where feed intake and nutrient digestibility were determined. The experimental design was a completely randomised design. Goats were individually housed in pens (1·5 m2/goat) with free access to water and offered the experimental diets to meet their nutrient requirements according to NRC (2007) recommendations. Adjustments were made to the diets to ensure collection of orts.
Basal diet fed to the goats contained 500 g of Egyptian berseem clover (Trifolium alexandrinum) and 500 g of concentrate feed mixture [containing (per kg DM basis): 250 g undecorticated cotton seed meal, 350 g wheat bran, 300 g maize, 30 g rice bran, 30 g molasses, 20 g limestone, 10 g urea and 10 g salt]. The basal diet and ingredients were previously reported by Kholif et al. (Reference Kholif, Matloup, Morsy, Abdo, Abu Elella, Anele and Swanson2017a, Reference Kholif, Morsy, Matloup, Anele, Mohamed and El-Sayedb) as the control diet. The basal diet contained 523 g DM (per kg wet material) and (per kg DM): 891 g OM, 142 g CP, 34 g ether extract, 354 g non-structural carbohydrates (NSC), 361 g neutral detergent fibre (NDF), and 238 g acid detergent fibre (ADF).
Goats were fed one of three diets. As previously noted, the control diet consisted of concentrate feed mixture and berseem clover (1 : 1 DM basis). The other two diets consisted of the control diet +10 g/goat daily dried mustard seeds (mustard treatment), or the control diet +10 g/goat daily cumin seeds (cumin treatment).
Diets were offered to each goat individually at 08:00 and 16:00 h in two equal portions. Daily allocation of cumin and mustard seeds for each goat were provided individually in 100 g concentrate before morning feeding at 08:00 h to assure their intake. Feed samples of berseem clover and concentrates mixture were taken daily, composited weekly and dried at 60 °C in a forced-air oven for 48 h and ground to pass a 1-mm screen using a Wiley mill, after which the ground samples were stored for chemical analyses.
Nutrient digestibility and chemical analysis
Feed intake was recorded daily by weighing feeds offered and orts from the previous day. Three digestibility trials were conducted at the 4th, 8th and 12th weeks of the experiment (7 days for each collection period), in which acid insoluble ash was used as an internal indigestibility marker, and coefficients of digestion were calculated as described in Kholif et al. (Reference Kholif, Morsy, Matloup, Anele, Mohamed and El-Sayed2017b).
Feed, orts, and faecal samples were ground through a 1-mm screen using a Wiley mill (Arthur H. Thomas, Philadelphia, PA, USA), and analysed for DM (method ID 930·15), ash (method ID 942·05), N (method ID 954·01), and ether extract (method ID 920·39), according to AOAC (1997) official methods. Neutral detergent fibre was determined by the procedure of Van Soest et al. (Reference Van Soest, Robertson and Lewis1991) without the use of alpha amylase but with sodium sulphite. Acid detergent fibre was analysed according to AOAC (1997; method ID 973·18). Lignin was analysed by solubilisation of cellulose with sulphuric acid in the ADF residue according to Van Soest et al. (Reference Van Soest, Robertson and Lewis1991). Non-structural carbohydrates [NSC = 1000 − (NDF + CP + EE + ash)] and OM (OM = 1000 − ash) were calculated (Kholif et al. Reference Kholif, Morsy, Matloup, Anele, Mohamed and El-Sayed2017b).
The chemical constituents of mustard and cumin seeds were analysed as described in Kholif et al. (Reference Kholif, Matloup, Morsy, Abdo, Abu Elella, Anele and Swanson2017a) at the Central Laboratory of National Research Centre using a Thermo Scientific Ultra/ISQ Single Quadrupole GC-MS system.
Sampling and analysis of rumen fluid
On the 7th day of the 8th and 12th weeks of the experiment, ruminal contents were sampled at 3 h post morning feeding to determine pH and concentration of fermentation end-products. Ruminal contents were collected once by using a stomach tube and hand pump. To avoid saliva contamination of ruminal content, the first 50 ml of the rumen fluid sample was discarded. Composite samples taken from each goat were strained through 4 layers of cheesecloth. The pH of ruminal fluid was measured immediately using a pH meter (HI98127 pHep®4 pH/Temperature Tester, Hanna® instrument, Italy).
A subsample of 5 ml was preserved in 5 ml of 0·2 m HCl for ammonia-N analysis and 0·8 ml of ruminal fluid was mixed with 0·2 ml of a solution containing 250 g of metaphosphoric acid/L for short chain FA (SCFA) analysis. All samples were stored at −20 °C until laboratory analyses. Concentration of ruminal ammonia-N was determined according to AOAC (1997; method ID 954·01). Total SCFA concentration in the samples was determined by titration, after steam distillation of a 4 ml sample. Proportions of the individual SCFA were measured by gas-liquid chromatography (Varian 3700; Varian Specialties Ltd, Brockville, Ontario, Canada). The separation process was carried out with a capillary column (30 m × 0·25 mm internal diameter, 1-mm film thickness, Supelco Nukol; Sigma–Aldrich, Mississauga, ON, Canada) with a flame ionisation detection.
Sampling and analysis of blood serum
On the final day of the 8th and 12th weeks, blood samples (10 ml) were taken 4 h after feeding from the jugular vein of each goat into a clean dry tube, without anticoagulants. Blood samples were centrifuged at 4000 g for 20 min. Serum was separated into 2-ml clean dried Eppendorf tubes and frozen at −20 °C until analysis. Blood serum samples were calorimetry analysed for concentrations of total protein, albumin, urea-N, glutamate-oxaloacetate transaminase (GOT), glutamate-pyruvate transaminase (GPT), glucose, and cholesterol using specific kits (Stanbio Laboratory, Boerne, Texas, USA) using a T80 UV/Vis spectrometer (PG instrument Ltd, Lutterworth, UK) following manufacturer instructions. Globulin concentration was calculated by subtracting albumin values from their corresponding total protein values (Kholif et al. Reference Kholif, Morsy, Matloup, Anele, Mohamed and El-Sayed2017b).
Milk sampling, milk composition and fatty acids analysis
Without oxytocin, and after removing the kids, goats were milked by hand twice daily at 09:00 and 21:00 h on the 4th, 8th, and 12th weeks, and samples (100 g/kg of recorded milk yield) were collected at each milking. A mixed sample of milk (proportional to amounts produced in the morning and evening) was taken daily. Milk samples were analysed for total solids, fat, protein, and lactose using infrared spectrophotometry (Foss 120 Milko-Scan, Foss Electric, Hillerød, Denmark). Ash content of milk was determined after heating in a muffle furnace at 550 °C for 8 h. Fatty acids in milk were determined at the Central Service Unit, National Research Centre (Egypt) using FA methyl esters prepared by base-catalysed methanolysis of the glycerides (NaOH in methanol) according to International Standards of International Dairy Federation (Brussels, Belgium) using an Agilent 19091J-413 HP-5 column containing 5% phenyl methyl siloxane (30 m × 0·32 mm i.d., df = 0·25 µm; Agilent, USA) on a gas chromatography (model 6890, Hewlett-Packard, Palo Alto, CA) equipped with a flame ionisation detector.
Average yields (g/d) of each milk component were calculated for each individual goat by multiplying milk yield by the component content (g/kg) of milk. The gross energy content in milk was calculated according to Tyrell & Reid (Reference Tyrell and Reid1965) equation. Milk energy output (MJ/d) was then calculated as milk energy (MJ/kg) × milk yield (kg/d). Energy corrected milk (ECM) was calculated according to Sjaunja et al. (Reference Sjaunja, Baevre, Junkkarinen, Pedersen and Setala1991) equation
Statistical analyses
Data for DM intake, apparent nutrients digestibility, milk characteristics, ruminal fermentation and blood profile were analysed using the PROC MIXED procedure of SAS (SAS Inst. Inc. Cary, NC) with week as a repeated measures and individual animal as the experimental unit. The model included the effect of treatment, week, and the treatment × week interaction. Two covariance structures were considered in the REPEATED statement in PROC MIXED: compound symmetry (cs) and auto-regressive (AR(1)). The error structure with the lowest Akaike information criteria fit statistic was selected for the model. When the treatment F-test was significant at P < 0·05, means were then compared by applying the probability of difference option of the least squares means statement and PDIFF option.
Results
Seeds active compounds
A total of 40 compounds were identified in the extract of mustard seeds, ranging from C6 to C45 compounds (Table 1). Retention time and mass spectral comparison identified 30 compounds in the seeds of cumin; they were C8 to C36 compounds (Table 2).
Table 1. Principal identified phytoconstituents of mustard seeds (Brassica juncea) extract by GC-MS analysis
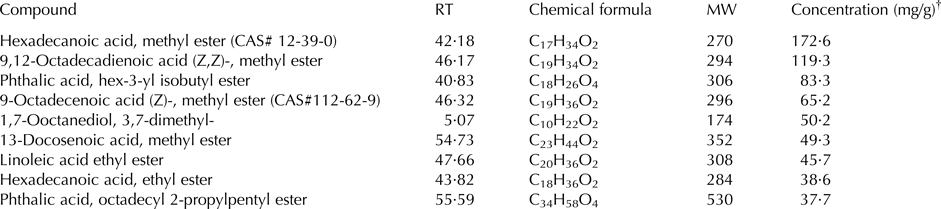
RT, retention time (min); MW, molecular weight of the compound (g/mol); CAS, Chemical Abstracts Service number.
† Concentration based on the total areas of the identified peaks.
Table 2. Principal identified phytoconstituents of cumin seeds (Cuminum cyminum) extract by GC-MS analysis
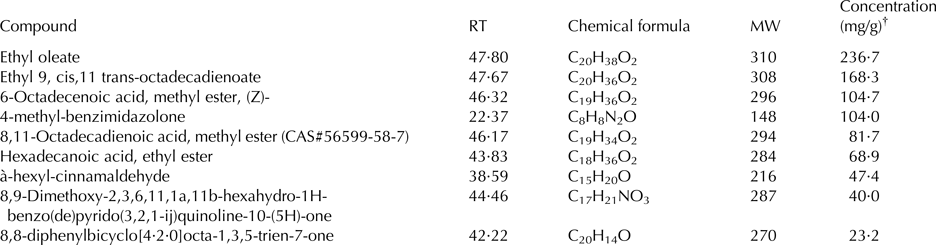
RT, retention time (min); MW, molecular weight of the compound (g/mol); CAS, Chemical Abstracts Service number.
† Concentration based on the total areas of the identified peaks.
Feed intake and nutrient digestibility
Week and treatment × week effects were not significant on feed intake and nutrient digestibility (Table 3). Moreover, no effect was observed with feeding mustard and cumin treatments on DM intake. Digestibilities of DM, OM, NSC, NDF and ADF were greater (P < 0·05) with mustard and cumin treatments compared with the control treatment. Goats fed cumin diet had greater OM (P < 0·001) digestibility compared with mustard treatment.
Table 3. Feed intake, and nutrients digestibility of lactating Damascus goats (n = 5 goats per treatment) fed a basal diet supplemented with mustard or cumin seeds
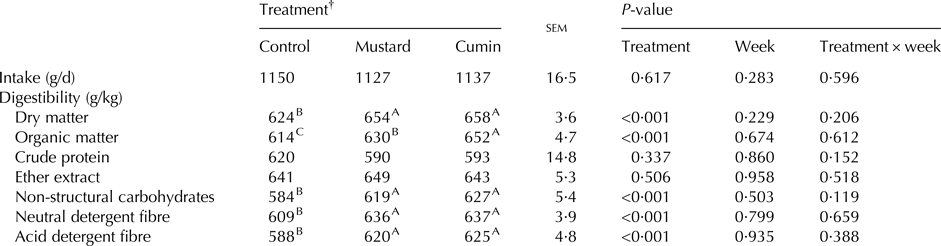
Within rows means bearing different superscripts differ significantly: A, B, C at P < 0·01. P-value is the observed significance level of the F-test for treatment; sem, standard error of the mean.
† The basal diet based on concentrates feed mixture and fresh Trifolium alexandrinum at 1 : 1 (DM basis) without (Control treatment) or with addition of mustard seeds (Mustard treatment) or cumin seeds (Cumin treatment) at 10 g/goat/d.
Ruminal fermentation and blood measurements
No week or treatment × week effects were observed for ruminal fermentation (Table 4). Besides, ruminal pH and butyrate concentration were not affected with feeding mustard and cumin treatments. On the contrary, greater concentrations of SCFA (P < 0·001), propionate (P = 0·002), and proportional propionate (P = 0·002); and lower ammonia-N (P = 0·002) and acetate (P = 0·03) concentrations were observed with feeding mustard and cumin treatments compared with the control treatment.
Table 4. Rumen fermentation of lactating Damascus goats (n = 5 goats per treatment) fed a basal diet supplemented with mustard or cumin seeds

Within rows means bearing different superscripts differ significantly: A, B, C at P < 0·01; a, b at P < 0·05. P-value is the observed significance level of the F-test for treatment; sem, standard error of the mean.
† The basal diet based on concentrates feed mixture and fresh Trifolium alexandrinum at 1 : 1 (DM basis) without (Control treatment) or with addition of mustard seeds (Mustard treatment) or cumin seeds (Cumin treatment) at 10 g/goat/d.
No difference was noted for serum albumin/globulin ratio, urea-N, and GPT concentrations as a result of the treatments. Both cumin and mustard treatments showed greater (P < 0·05) concentrations of serum total proteins, globulin, and glucose and lower (P < 0·01) serum GOT and cholesterol. Besides, higher (P < 0·01) globulin and glucose concentrations were observed with the inclusion of cumin compared with mustard (Table 5).
Table 5. Blood chemistry of lactating Damascus goats (n = 5 goats per treatment) fed a basal diet supplemented with mustard or cumin seeds
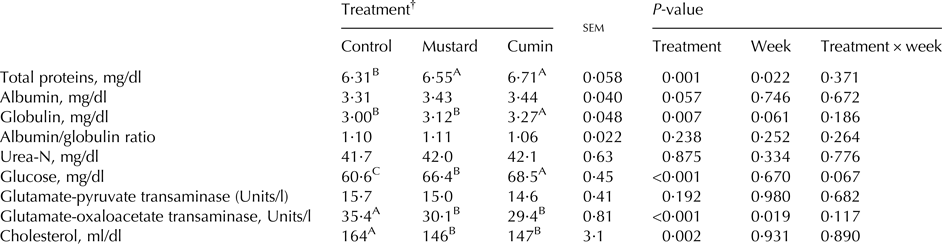
Within rows means bearing different superscripts differ significantly: A, B, C at P < 0·01. P-value is the observed significance level of the F-test for treatment; sem, standard error of the mean.
† The basal diet based on concentrates feed mixture and fresh Trifolium alexandrinum at 1 : 1 (DM basis) without (Control treatment) or with addition of mustard seeds (Mustard treatment) or cumin seeds (Cumin treatment) at 10 g/goat/d.
Milk yield and composition
With no significant treatment × week interaction, sampling week effects were significant (P < 0·05) for yield and concentrations and yields of milk component (Table 6). Dietary inclusion of mustard and cumin resulted in greater milk yield (P = 0·005), ECM (P = 0·001), total solids (P = 0·001), solids not fat (P = 0·003), fat (P < 0·001), lactose (P < 0·001), and ash (P = 0·046) compared with the control treatment. In addition, milk contents of total solids, solids not fat, fat and lactose were greater (P < 0·05) with the inclusion of mustard and cumin seeds. Greater milk fat content (P = 0·019) was noted in cumin treatment compared with the mustard treatment. Milk efficiency as milk/DM intake (P = 0·011), and ECM/DM intake (P = 0·003) were greater with the mustard and cumin treatments compared with the control treatment, with no difference (P > 0·05) between mustard and cumin treatments.
Table 6. Milk yield and composition of lactating Damascus goats (n = 5 goats per treatment) fed a basal diet supplemented with mustard or cumin seeds
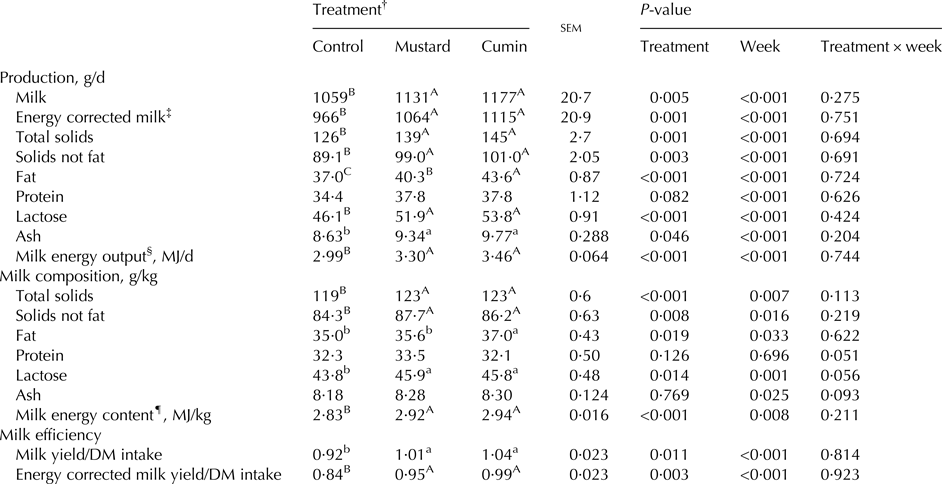
Within rows means bearing different superscripts differ significantly: A, B, C at P < 0·01; a, b at P < 0·05. P-value is the observed significance level of the F-test for treatment; sem, standard error of the mean.
† The basal diet based on concentrates feed mixture and fresh Trifolium alexandrinum at 1 : 1 (DM basis) without (Control treatment) or with addition of mustard seeds (Mustard treatment) or cumin seeds (Cumin treatment) at 10 g/goat/d.
‡ Energy correct milk (kg/d) = milk (kg/d) × [38·3 × fat (g/kg) + 24·2 × protein (g/kg) + 16·54 × lactose (g/kg) + 20·7]/3140 (Sjaunja et al. Reference Sjaunja, Baevre, Junkkarinen, Pedersen and Setala1991).
§ Milk energy output (MJ/d) was then calculated as milk energy (MJ/kg) × milk yield (kg/d).
¶ Milk energy content (MJ/kg) = 4·184 × 2·204 × [(41·63 × fat (g/100 g) + 24·13 × protein (g/100 g) + 21·60 × lactose (g/100 g) − 11·72)/1000] (Tyrell & Reid, Reference Tyrell and Reid1965).
Milk fatty acids profile
Week and treatment × week effects did not affect milk fatty acids profile (Table 7). Both treatments (mustard and cumin) lowered (P < 0·05) milk contents of C8:0, C12:0, C17:0, C18:0, and C18:1 n9C FA. Greater (P < 0·05) C14:1, C15:0, C16:1, cis-9, trans-11 C18:2, and C18:3 n-6 FA concentrations were noted for cumin and mustard treatments vs. the control treatment. Goats supplemented with cumin had greater (P < 0·05) C15:0, C16:1, trans-10, cis-12 C18:2, and C18:3 n-3 concentrations compared with mustard treatment.
Table 7. Fatty acid profile (g/100 g total fatty acids) in milk of lactating Damascus goats (n = 5 goats per treatment) fed a basal diet supplemented with mustard or cumin seeds
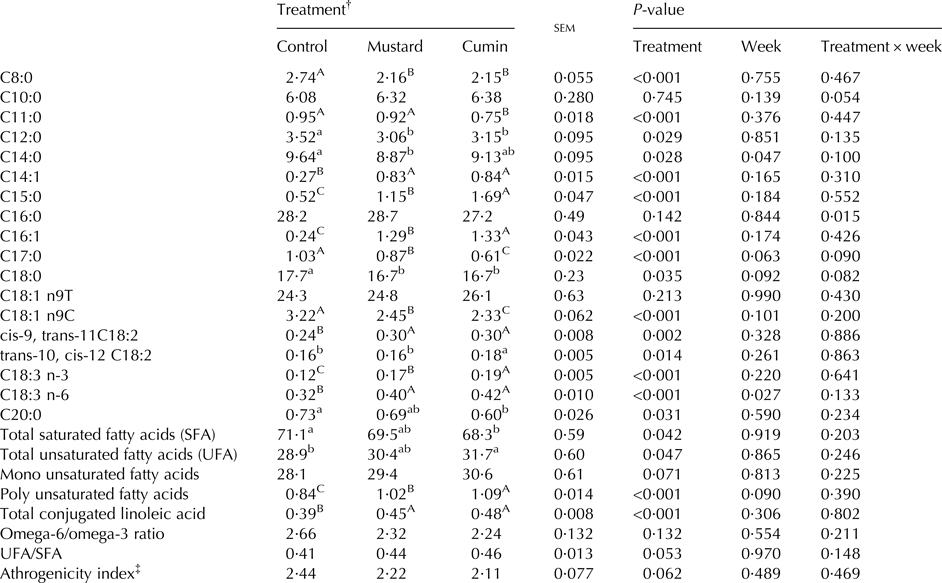
Within rows means bearing different superscripts differ significantly: A, B, C at P < 0·01; a, b at P < 0·05. P-value is the observed significance level of the F-test for treatment; sem, standard error of the mean.
† The basal diet based on concentrates feed mixture and fresh Trifolium alexandrinum at 1 : 1 (DM basis) without (Control treatment) or with addition of mustard seeds (Mustard treatment) or cumin seeds (Cumin treatment) at 10 g/goat/d.
‡ Calculated according to Ulbricht & Southgate (Reference Ulbricht and Southgate1991): athrogenicity index = (C12:0 + 4 × C14:0 + C16:0)/∑ of total unsaturated fatty acids.
There was no effect on mono unsaturated FA (MUFA), omega-6/omega-3, UFA/SFA and athrogenicity index but concentration of total CLA and polyunsaturated FA (PUFA) were greater (P < 0·001) with mustard and cumin supplementation. Compared with the control treatment, the cumin treatment had greater UFA (P = 0·047).
Discussion
Results of GC-MS analyses indicated that the chemical constituents of cumin and mustard differed quantitatively and qualitatively. The GC-MS analysis showed that the principal compounds of the mustard seed were hexadecanoic acid, methyl ester and 9,12-octadecadienoic acid (Z,Z)-, methyl ester. On the contrary, ethyl oleate, ethyl 9, cis,11 trans-octadecadienoate, -octadecenoic acid, methyl ester, (z)-, and 4-methyl-benzimidazolone were the principal compounds in cumin seed. No information is available about the biological activity of most of these phytoconstituents; however, hexadecanoic acid, methyl ester has some antibacterial, antifungal properties and possesses antioxidant activity. Rajeswari et al. (Reference Rajeswari, Murugan and Mohan2013) reported that the biological activity of hexadecanoic acid, methyl ester includes 5-α reductase inhibitor, haemolytic, hypocholesterolemic, antioxidant nematicide, and pesticide. Some information are available about the biological activity of octadecadienoic acid as hypocholesterolemic, hepatoprotective, and 5-α reductase inhibitor (Rajeswari et al. Reference Rajeswari, Murugan and Mohan2013). Such biological activities may be responsible for the positive effects of feeding mustard and cumin seeds to goats.
Feed intake and digestibility
Dietary inclusion of mustard and cumin seeds did not affect feed intake, revealing no negative effect of the seeds on diet acceptability (Moss, Reference Moss1975; Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013). This confirms the hypothesis that goats have high tolerance toward the bitter taste of plant secondary compounds (Tripathi & Mishra, Reference Tripathi and Mishra2007). Consistent with the present results, Miri et al. (Reference Miri, Tyagi, Ebrahimi and Mohini2013) observed that feeding cumin seed extract at 1·27 and 2·53% DM intake had no effect on DM intake of lactating goats. However, Ghafari et al. (Reference Ghafari, Shahraki, Nasrollahi, Amini and Beauchemin2015) observed increased feed intake with increasing levels of cumin seed in the diet of lactating cows. The inconsistence may be as a result of different doses and animal species. The lack of differences in feed intake with feeding mustard reveals that the allyl isothiocyanate contained in mustard seeds (Moss, Reference Moss1975) did not adversely affect intake in goats. The present results could not be compared. The understanding of possible mechanisms driving the lack of difference in feed intake and improved feed nutrient digestibility and ruminal fermentation when adding cumin or mustard seeds to the diet is limited.
Enhanced nutrient digestibility observed with cumin and mustard inclusion may have resulted from improved ruminal fermentation with feeding mustard and cumin seeds. The presence of secondary metabolites in the seeds of these plants may have optimised the availability and activity of the rumen microflora and other ruminal functions, resulting in improved feed nutrients digestion by goats (Salem et al. Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014; Kholif et al. Reference Kholif, Morsy, Gouda, Anele and Galyean2016). High levels of secondary metabolites have detrimental effects on feed utilisation (Salem et al. Reference Salem, Kholif, Elghandour, Buendía, Mariezcurrena, Hernandez and Camacho2014). However, recent reports stated that low and medium levels of secondary metabolites have some positive effects on ruminal fermentation and productivity in vivo (Cedillo et al. Reference Cedillo, Kholif, Salem, Elghandour, Vázquez, Alonso, Barbabosa, Chagoyán and Reyna2015; Kholif et al. Reference Kholif, Gouda, Anele and Galyean2018) and in vitro (Chaudhry & Khan, Reference Chaudhry and Khan2012). Ruminal microflora has the ability to degrade and utilise low and moderate concentrations of the secondary metabolites without negative effects on animal performance and ruminal fermentation (Kholif et al. Reference Kholif, Gouda, Morsy, Salem, Lopez and Kholif2015, Reference Kholif, Gouda, Anele and Galyean2018).
Ruminal fermentation measurements
It is well documented that tannins can bind protein and reduce ruminal protein degradability and plant cell wall digestion (Bodas et al. Reference Bodas, Prieto, García-González, Andrés, Giráldez and López2012). However, in the present study CP digestibility was not affected with feeding mustard and cumin diets, while dietary fibre fractions were increased. This may be a reflection of the appropriate levels of tannins and other secondary metabolites with feeding 10 g of cumin and mustard seeds.
Greater concentrations and molar proportions of propionate and acetate observed with feeding mustard and cumin are considered beneficial in ruminant nutrition and dairy production (Kholif et al. Reference Kholif, Morsy, Gouda, Anele and Galyean2016). Propionic acid is the primary gluconeogenic SCFA that affect lactose biosynthesis, while acetate is the precursor for milk fat biosynthesis. Most studies with feeding secondary metabolites containing plants indicated increased proportion of propionate and a reduction in proportion of acetate (Bodas et al. Reference Bodas, Prieto, García-González, Andrés, Giráldez and López2012). This may be related to the positive effects of secondary metabolites in inhibiting Gram-positive bacteria (usually acetate producers) and favouring propionate producing bacterial species; thereby resulting in increased accumulation of propionate in the rumen (Wallace et al. Reference Wallace, McEwan, McIntosh, Teferedegne and Newbold2002). Miri et al. (Reference Miri, Ebrahimi and Tyagi2015) observed that feeding goats on diets containing cumin seed extract had no effect on ruminal total SCFA concentration and molar proportion of individual SCFA. To the best of our knowledge, only Miri et al. (Reference Miri, Ebrahimi and Tyagi2015) studied the effect of cumin on ruminal SCFA concentration in lactating goats. However, in an in vitro experiment, Chaudhry & Khan (Reference Chaudhry and Khan2012) reported that addition of cumin seed powder to incubated substrate was associated with the increased proportion of acetate and lower molar proportion of propionate. Inclusion of cumin produced more SCFA in the rumen of goats than mustard treatment. This may be due to the presence of different phytoconstituents between cumin and mustard seeds. No information is available about the biological activity of individual phytoconstituents of the seeds to compare the present results.
Ruminal ammonia-N concentration ranged from 25·3 to 28·1 g/l which falls within the range reported for maximal microbial growth and activity (Satter & Slyter, Reference Satter and Slyter1974). The presence of secondary metabolites above specific levels usually depresses ruminal ammonia N production (Bodas et al. Reference Bodas, Prieto, García-González, Andrés, Giráldez and López2012), which is evidence of improved absorption of feed amino acid. In their review, Bodas et al. (Reference Bodas, Prieto, García-González, Andrés, Giráldez and López2012) reported two mechanisms to explain the reduction of ruminal ammonia-N production as: (1) the reduction in peptidolytic activity, (2) inhibition of hyper ammonia-producing bacteria.
Blood metabolites
Results of blood metabolites in the present study were within the reference ranges reported by Boyd (Reference Boyd1984). The greater concentrations of serum total proteins, and globulin is an important indicator for improved nutritional status and lactational performance of animals (Kholif et al. Reference Kholif, Matloup, Morsy, Abdo, Abu Elella, Anele and Swanson2017a, Reference Kholif, Morsy, Matloup, Anele, Mohamed and El-Sayedb).
Lack of effect on blood urea-N reveals the minimal protein catabolism, and normal kidney function. However feeding cumin and mustard lowered ruminal ammonia-N concentration, blood urea-N concentration wasn't affected. The reason for this result is unclear so one can only speculate. Lowering ruminal ammonia without affecting the blood urea-N may be an indicator of higher conversation of ruminal ammonia-N to microbial protein. Blood urea-N comes from two entry points; ruminal degradation of protein, and the degradation of protein by tissues. Moreover, many other factors can affect blood urea-N concentration including energy intake, water intake, protein supply, liver and kidney function, and milk production (Hristov et al. Reference Hristov, Etter, Ropp and Grandeen2004).
Liver activity and health (measured as GOT and GPT) were not affected with feeding mustard and cumin seeds. Concentrations of GPT and GOT are important indicators of liver activity, function and hepatotoxicity suggesting absent of pathological lesions in the liver (Pettersson et al. Reference Pettersson, Hindorf, Persson, Bengtsson, Malmqvist, Werkström and Ekelund2008).
Greater serum glucose concentrations with feeding mustard and cumin may be due to increased rumen propionate as a result of increased OM digestibility. Interestingly, serum glucose of goats fed cumin and mustard treatments followed similar trend as OM digestibility and milk yield. Ghafari et al. (Reference Ghafari, Shahraki, Nasrollahi, Amini and Beauchemin2015) noted that glucose concentrations were not affected in cows fed diets supplemented with cumin seeds. The antioxidant characteristics of mustard and cumin seeds may be a reason for the observed increase in serum glucose.
The decreased cholesterol level in the present study is consistent with previously reported cholesterol-lowering effects of cumin (Zare et al. Reference Zare, Heshmati, Fallahzadeh and Nadjarzadeh2014) in overweight and obese women at 3 g/d cumin powder. However, the mechanism by which mustard and cumin seeds supplementation reduced cholesterol has not been fully explored.
Milk production, composition and fatty acids profile
Expectedly, sampling time affected milk production and component yields. This is consistent with the observations of Rojo-Rubio et al. (Reference Rojo-Rubio, Kholif, Salem, Mendoza, Elghandour, Vazquez-Armijo and Lee-Rangel2016) who reported a correlation between milk production and sampling date in goats. One of the most important result observes with mustard and cumin seeds inclusion in this study was the greater milk production (actual yield increased by 6·8 and 11·1%, while the ECM increased by 10·1 and 15·4% for mustard and cumin treatments, respectively). Enhancing nutrient digestibility and ruminal fermentation with feeding mustard and cumin seeds are the main reasons for observed greater milk production; however, feed intake was not affected. Similarly, we noted improvement in milk production efficiency (milk/DM intake; by 9·8 and 13·0%, and ECM/DM intake; by 13·1 and 17·9%) for mustard and cumin seeds, respectively. Higher milk lactose can be another reason for increased milk production with feeding mustard and cumin diets, as reported by Rigout et al. (Reference Rigout, Hurtaud, Lemosquet, Bach and Rulquin2003). Moreover, the galactopoietic property of mustard and cumin may explain the increased milk production (Bhatt et al. Reference Bhatt, Singh and Ali2009) as a result of stimulated hormonal secretion in mammals (Kumar et al. Reference Kumar, Mehla and Dang2008). Both mustard and cumin elevated milk lactose content. Propionate is the precursor for gluconeogenesis and lactose synthesis, and increasing glucogenic precursors has a favourable effect on milk lactose content.
About 5·7% increase in milk fat content in cumin treatment may be due to greater ruminal acetate production with feeding cumin seeds. Milk fat content and FA composition are sensitive to dietary manipulation than other milk constituents. Ghafari et al. (Reference Ghafari, Shahraki, Nasrollahi, Amini and Beauchemin2015) reported that fat content and yield of cows were not affected with inclusions of cumin seeds in the diet. Miri et al. (Reference Miri, Tyagi, Ebrahimi and Mohini2013) observed no difference in milk composition but recorded about 13% greater milk yield with feeding cumin seed extract to lactating goat. The discrepancy between the present study and others may be due to diet composition (320 g NDF in Ghafari et al. (Reference Ghafari, Shahraki, Nasrollahi, Amini and Beauchemin2015), and 468 g NDF in Miri et al. (Reference Miri, Tyagi, Ebrahimi and Mohini2013) vs. 361 g/kg DM in the present study), or due to the inclusion level (up to 1·2% of DM intake in Ghafari et al. (Reference Ghafari, Shahraki, Nasrollahi, Amini and Beauchemin2015), 1·27 and 2·53% DM intake in Miri et al. (Reference Miri, Tyagi, Ebrahimi and Mohini2013) vs. 0·9% in the present study). Greater inclusion levels in the other studies compared with the present study may have affected ruminal FA biohydrogenation due to the antibacterial property (Tajkarimi et al. Reference Tajkarimi, Ibrahim and Cliver2010), and blocking a specific reaction in biohydrogenation pathway (Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013), resulting in different milk fat concentrations. Ruminal fermentation can be another factor causing the inconstancy between the present study and others; however, both of them didn't study the ruminal fermentation with feeding cumin. Almost no information is available on mustard seeds to be compared with the present results.
Inclusion of cumin lowered milk individual and total SFA (by about 3·9%) and increased individual and total UFA (by about 9·7%), and total CLA (by about 23·1%). Besides, mustard seeds inclusion increased total CLA of milk by about 15·4%. These effects are very beneficial from the point of view of human nutrition for the prevention of cardiovascular disease for milk consumers. Increasing milk UFA content is very important as UFA are bioregulators of many cellular processes and linked to the development and functionality of the immune system. Greater PUFA content of milk in goats receiving mustard and cumin is consistent with a previous report (Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013).
The difference observed between cumin and mustard on milk FA is related with the different phytoconstituents. In their experiment, Miri et al. (Reference Miri, Tyagi, Ebrahimi and Mohini2013) observed that cumin seed extract inclusion in the diet of lactating goats altered milk FA profile, lowered SFA and increased PUFA, MUFA, UFA, and UFA/SFA. It was assumed that ruminal biohydrogenation has the ability to convert UFA to SFA; however, this was not clear in the present experiment. Secondary compounds of plants exert an antimicrobial effect on biohydrogenating bacterial species in the rumen (Bodas et al. Reference Bodas, Prieto, García-González, Andrés, Giráldez and López2012) resulting in accumulation of biohydrogenation intermediates and enhancement of milk PUFA and CLA content (Miri et al. Reference Miri, Tyagi, Ebrahimi and Mohini2013; Kholif et al. Reference Kholif, Gouda, Morsy, Salem, Lopez and Kholif2015, Reference Kholif, Morsy, Gouda, Anele and Galyean2016). This statement is true for cumin and mustard seeds in the present study, which resulted in the accumulation of UFA in the milk. Harfoot & Hazlewood (Reference Harfoot, Hazlewood, Hobson and Stewart1997) reported that CLA are produced as intermediate products in the biohydrogenation of linoleic acid to stearic acid by certain groups of ruminal bacteria. However, the main biologically active isomer of CLA, cis-9 trans-11 CLA or cis-9, trans-11 C18:2, also forms endogenously in mammary gland from vaccenic acid by the action of delta-9 desaturase enzyme (Griinari et al. Reference Griinari, Corl, Lacy, Chouinard, Nurmela and Bauman2000). The GC-MS analysis of cumin and mustard seeds showed that they contain some phenolic compounds which have the ability to affect FA metabolism during ruminal biohydrogenation. Moreover, cuminaldehyde, the major constituent of cumin essential oils, has been reported to be bound with tyrosinase, aldose reductase and α- glucosidase enzymes to form a complex causing inhibited enzymes activities (Kubo & Kinst-Hori, Reference Kubo and Kinst-Hori1998). Another explanation can be based on the observations of Frankič et al. (Reference Frankič, Voljč, Salobir and Rezar2009) who reported that cumin has the ability to enhance lipids absorption in the small intestine.
Conclusions
Daily addition of mustard and cumin seeds at 10 g/goat did not affect nutrients intake, but enhanced nutrient digestibility, ruminal fermentation and milk yield by about 6·8 and 11·1%. Moreover, the seeds positively affected milk FA profile as the relative percentage of UFA and CLA were increased whereas SFA were lowered; with better performance with the cumin seeds than mustard seeds. Based on the present study, there are potential benefits of cumin and mustard seeds inclusion in the diet of lactating Damascus goats.









