Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Bhaskar, G.V.
Campanella, O.H.
and
Munro, P.A.
1993.
Effect of agitation on the coagulation time of mineral acid casein curd: Application of Smoluchowski's orthokinetic aggregation theory.
Chemical Engineering Science,
Vol. 48,
Issue. 24,
p.
4075.
Bringe, N.A.
and
Kinsella, J.E.
1993.
Calcium chloride, temperature, preheat treatments and pH affect the rate of acid-induced aggregation of casein.
Food Hydrocolloids,
Vol. 7,
Issue. 2,
p.
113.
Ould Eleya, M.M.
Desobry Banon, S.
and
Hardy, J.
1995.
A Comparative Study of pH and Temperature Effects on the Acidic Coagulation of Milks from Cows, Goats, and Sheep.
Journal of Dairy Science,
Vol. 78,
Issue. 12,
p.
2675.
Goddard, Simon J.
and
Augustin, Mary-Ann
1995.
Formation of acid-heat-induced skim milk gels in the pH range 5·0–5·7: effect of the addition of salts and calcium chelating agents.
Journal of Dairy Research,
Vol. 62,
Issue. 3,
p.
491.
Tomasula, Peggy M.
Craig, James C.
Boswell, Richard T.
Cook, Richard D.
Kurantz, Michael J.
and
Maxwell, Maryann
1995.
Preparation of Casein Using Carbon Dioxide.
Journal of Dairy Science,
Vol. 78,
Issue. 3,
p.
506.
Teo, Cheng Tet
Munro, Peter A.
and
Singh, Harjinder
1996.
Reversibility of shrinkage of mineral acid casein curd as a function of ionic strength, pH and temperature.
Journal of Dairy Research,
Vol. 63,
Issue. 4,
p.
555.
Kuo, Ching-Yun
Wang, Feng-Sheng
and
Lin, Chin-Wen
1996.
Factors affecting milk clotting activity of sweet leavening extract involved in coagulation of a yoghurt-like product.
Food Chemistry,
Vol. 55,
Issue. 2,
p.
129.
Tamime, A. Y.
and
Marshall, V. M. E.
1997.
Microbiology and Biochemistry of Cheese and Fermented Milk.
p.
57.
Lefebvre-Cases, E.
Gastaldi, E.
Vidal, V.
Marchessau, S.
Lagaude, A.
Cuq, J.-L.
and
De La Fuente, B. Tarodo
1998.
Identification of Interactions Among Casein Gels Using Dissociating Chemical Agents.
Journal of Dairy Science,
Vol. 81,
Issue. 4,
p.
932.
KEOGH, M.K.
and
O'KENNEDY, B.T.
1998.
Rheology of Stirred Yogurt as Affected by Added Milk Fat, Protein and Hydrocolloids.
Journal of Food Science,
Vol. 63,
Issue. 1,
p.
108.
Hofland, Gerard W.
van Es, Marco
van der Wielen, Luuk A. M.
and
Witkamp, Geert-Jan
1999.
Isoelectric Precipitation of Casein Using High-Pressure CO2.
Industrial & Engineering Chemistry Research,
Vol. 38,
Issue. 12,
p.
4919.
Slaćanac, V.
Novaković, P.
Petrak, T.
and
Kordić, J.
2000.
Application of mathematical models in milk coagulation process during lactic acid fermentation. Part I. Relation betwen enzymmatic and acidic milk coagulation.
Acta Alimentaria,
Vol. 29,
Issue. 3,
p.
241.
Lefebvre‐Cases, E.
La Fuente, B. Tarodo De
and
Cuq, J.L.
2001.
Effect of SDS on Casein Micelles: SDS‐Induced Milk Gel Formation.
Journal of Food Science,
Vol. 66,
Issue. 1,
p.
38.
Lefebvre‐cases, E.
DE LA Fuente, B. TARODO
and
Cuq, J.L.
2001.
Effect of SDS on Acid Milk Coagulability.
Journal of Food Science,
Vol. 66,
Issue. 4,
p.
555.
Cases, E.
Vidal, V.
and
Cuq, J.L.
2003.
Effect of K‐Casein Deglycosylation on the Acid Coagulability of Milk.
Journal of Food Science,
Vol. 68,
Issue. 8,
p.
2406.
Hofland, G. W.
Berkhoff, M. R.
Witkamp, G. J.
and
van der Wielen, L. A. M.
2003.
Dynamics of isoelectric precipitation of casein using sulfuric acid.
AIChE Journal,
Vol. 49,
Issue. 8,
p.
2211.
Matia-Merino, Lara
Lau, Ka
and
Dickinson, Eric
2004.
Effects of low-methoxyl amidated pectin and ionic calcium on rheology and microstructure of acid-induced sodium caseinate gels.
Food Hydrocolloids,
Vol. 18,
Issue. 2,
p.
271.
Cases, E.
Rampini, C.
and
Cayot, Ph.
2005.
Interfacial properties of acidified skim milk.
Journal of Colloid and Interface Science,
Vol. 282,
Issue. 1,
p.
133.
Tamime, A.Y.
and
Robinson, R.K.
2007.
Tamime and Robinson's Yoghurt.
p.
13.
Kök, M. Samil
2010.
Characterization of Galactomannan Stabilised Yogurt Drink Using Dynamic Rheology.
International Journal of Food Properties,
Vol. 13,
Issue. 1,
p.
209.
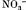 and SCN− bind to lysine, arginine and histidine groups and thereby decrease repulsive hydration forces between cationic casein micelles.
and SCN− bind to lysine, arginine and histidine groups and thereby decrease repulsive hydration forces between cationic casein micelles.

