Published online by Cambridge University Press: 01 June 2009
The rate of destruction of Bacillus subtilis spores by sodium hydroxide solutions in the range of atmospheric temperatures 34—82 °F was studied: (a) to provide information which might be used in practice for immersion cleaning of dairy utensils in sodium hydroxide solutions at low atmospheric temperatures, and (b) to examine the nature of the disinfection curves by slowing down the rate of disinfection. The results expressed as a three-dimensional graph showed that an increase in temperature from 34 to 82 °F had a more marked effect on spore destruction than an increase in concentration of sodium hydroxide from 1·5 to 5·0%. The overall effect could be expressed as
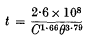
where t = time in hours for the 99% destruction of B. subtilis spores, C = concentration of NaOH (%, w/v) and θ = temperature, °F. Since the time for the 99% destruction of B. subtilis spores by a 2% NaOH solution at 34 °F is 5 times that of a 5% solution, immersion cleaning in cold climates might be assisted by increasing the concentration of NaOH from the normal 2–3 to 5%. Results in disinfection tests at low temperatures were more variable than had been observed in previous work at higher temperatures. These surprising results prevented a careful study of the nature of the disinfection curves and confirmed previous conclusions that such curves are basically sigmoid.