Introduction
The COVID-19 pandemic exposed two serious interrelated problems in the U.S. health care system – health disparities and loss of public trust. The U.S. clinical research enterprise faces the same issues, with the Tuskegee U.S. Public Health Service syphilis study and Henrietta Lacks’ loss of autonomy in the disposition of her cells painful, well-known examples that have had long-term negative impact on the American Black community’s trust in medical research, medical researchers, and willingness to participate in clinical studies [Reference Scharff, Mathews, Jackson, Hoffsuemmer, Martin and Edwards1,Reference Andrews and Davies2]. Moreover, trust plays a vital role in the willingness of individuals to participate in clinical studies, which is the core of the clinical research enterprise [Reference Viswanath, Bekalu, Dhawan, Pinnamaneni, Lang and McLoud3–Reference Yu, Bauermeister and Oyiborhoro5].
Taylor et al. recently performed a synthetic review of fifty years of research on health-related trust [Reference Taylor, Nong and Platt6]. They concluded that trust rests on a core belief “that individuals and institutions will act appropriately and perform competently, responsibly and in a manner considerate of our interests.” They pointed out two major obstacles to measuring trust and designing programs to increase trustworthiness: ambiguity in the definitions and methodological problems in empirical assessment of trustworthiness and trust. For example, some investigators have defined trustworthiness as “the quality of being trusted,” whereas others focus on the “quality of being deserving of trust.” Griffith et al. and Anderson and Griffith emphasized the subtle but important differences between low trust, distrust, and mistrust; low trust involves a reluctance to make oneself vulnerable at the hands of a person or organization, distrust is concern that another person or organization will attempt harmful behavior, and mistrust is a generalized skepticism based on historical injustice and systemic racism [Reference Griffith, Bergner, Fair and Wilkins7,Reference Anderson and Griffith8]. Benkert et al., proposed that mistrust is the tendency to distrust medical systems and personnel believed to represent the dominant culture [Reference Benkert, Cuevas, Thompson, Dove-Meadows and Knuckles9]. Mistrust was subcategorized into general trustworthiness, perceptions of discrimination, perceptions of deception, or perceptions of exploitation by Smirnoff et al. in their study of New Yorkers [Reference Smirnoff, Wilets and Ragin10]. They found that different demographic variables were associated with each of the subcategories in this paradigm, with, for example, being older being associated with viewing researchers as trustworthy; being African American, Latino, or having Spanish language preference being associated with having feelings of discrimination, having prior research experience or being African American being associated with perceiving researchers as deceptive, and having a high school education or less being associated with having feelings of exploitation.
There is a lack of agreement on the best measures of trust, with many different instruments proposed for measuring patient trust in physicians, ranging in granularity from 5 to 51 items, but with a lack of rigorous psychometric validation [Reference Taylor, Nong and Platt6]. Moreover, some tools focus on trust, whereas others concentrate on distrust or mistrust. More recently, as hospitals merged and developed into health systems, instruments were designed to measure trust in healthcare organizations, raising issues as to potential conceptual differences between trust in one’s own physician versus trust in physicians in general, and trust in organizations. Anderson and Griffith analyzed perceptions of whether a health system is considered worthy of trust, emphasizing the importance of the patient perceiving “that the organization or system has a material interest in optimizing the potential benefits of their health care and in minimizing potential harms” [Reference Anderson and Griffith8]. Figure 1 reprints their conceptual framework for understanding the complex interaction between trustworthiness and trust, and Figure 2 identifies elements that have been proposed to be important in being considered a trustworthy clinical research entity along with some of our own suggested elements [Reference Griffith, Jaeger, Bergner, Stallings and Wilkins11–Reference Kost, Lee and Yessis14]. Additional complexity emanates from consideration of other related trust issues, including physician trust in their patients, their colleagues, and their health care organizations. There are also differences in whether individuals trust a physician’s competence versus that physician’s values, with relatively minor differences between Black and White populations in the former, and rather larger differences in the latter [Reference Armstrong, Putt and Halbert15]. Similarly, differences in trust within a diverse group of patients tracks more closely to personal experience of discrimination rather than racial identity. Overlying all of these challenges is a meager empirical base for unequivocally demonstrating a compelling correlation between patient trust or institutional trustworthiness and objective clinical outcomes [Reference Taylor, Nong and Platt6,Reference Benkert, Cuevas, Thompson, Dove-Meadows and Knuckles9].
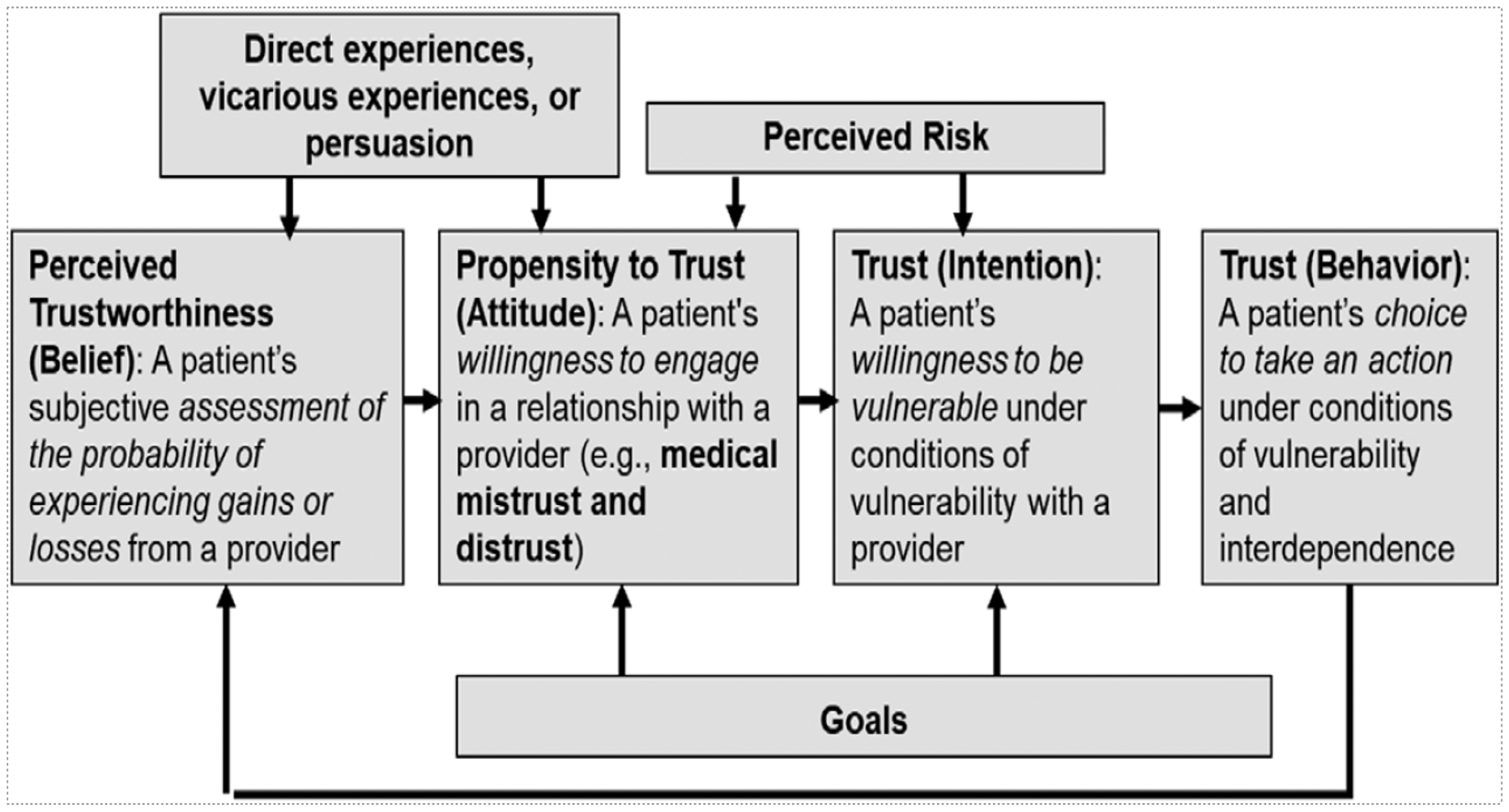
Figure 1. Conceptual modes proposed by Anderson and Griffith to explain the inter-relationship of trustworthiness and trust in health care organizations and systems. Reprinted with permission from their publication “Trustworthiness of health care organizations and systems,” The Milbank Quarterly, 100:345, 2022.
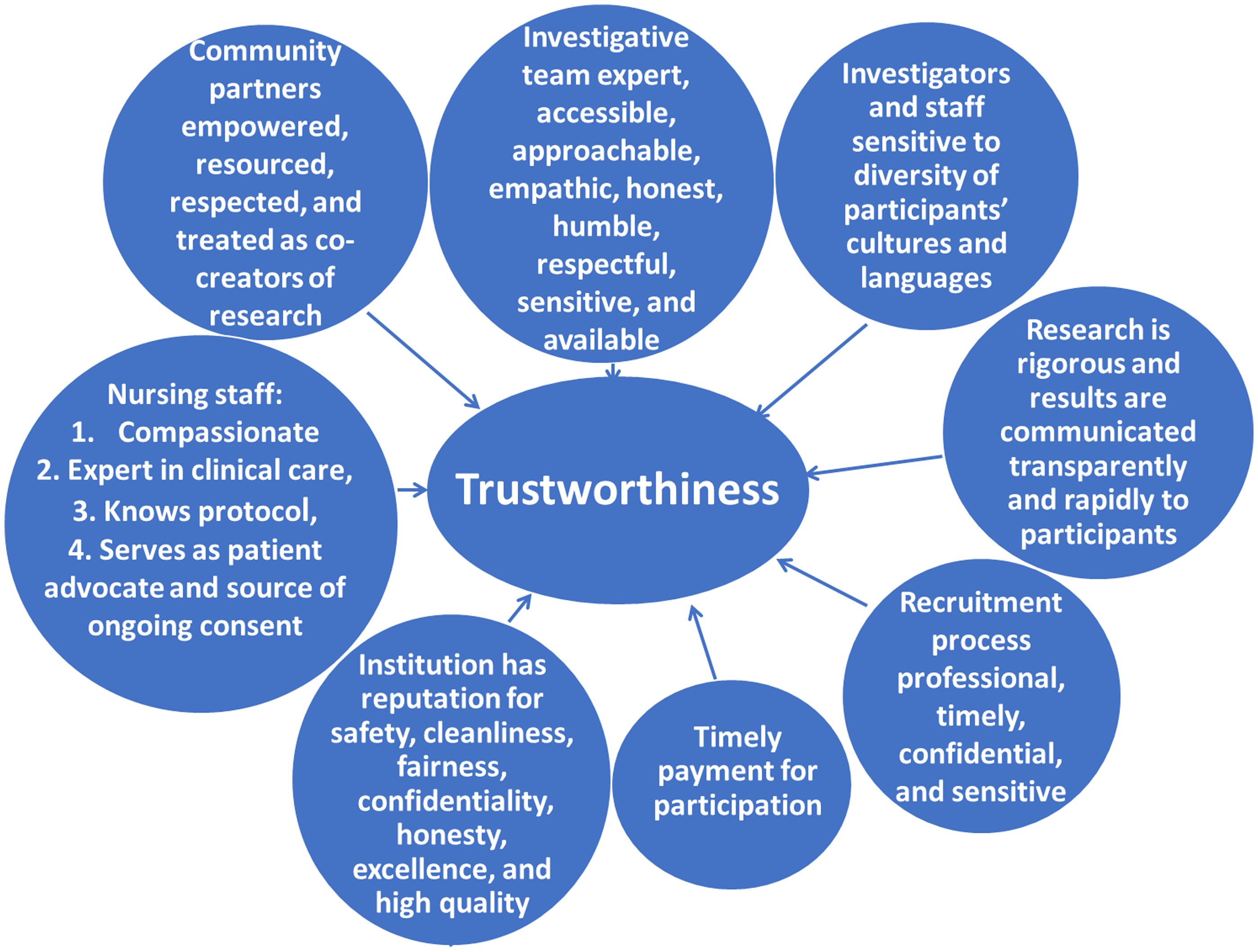
Figure 2. Elements proposed to impact whether a clinical research entity is considered worthy of trust [Reference Griffith, Jaeger, Bergner, Stallings and Wilkins11–Reference Kost, Lee and Yessis14].
The COVID-19 pandemic brought to the front the variability and, ultimately, the erosion of trust in public health recommendations, along with the consequences. The vaccination rate for demonstrably effective vaccines in the U.S. was disappointing, with nearly 20% of the population still not vaccinated [16], and the mortality from SARS-COV-2 infection in the U.S. being among the highest when compared to many other countries [Reference Msemburi, Karlinsky, Knutson, Aleshin-Guendel, Chatterji and Wakefield17]. Hostile refusal of measures to prevent infection from pandemics has a long history, extending back at least to resistance to variolation in response to 18 th -century outbreaks of smallpox [Reference Eichman and Bichianu18], and extending to resistance to Jenner’s smallpox vaccine in the 19th century [Reference Parodi and Martini19]. There have, however, also been periods of great public trust in public health recommendations, one example being the willingness of millions of New Yorkers to line up to be vaccinated against smallpox within weeks in 1947 in response to an index case in the city [Reference Sepkowitz20,Reference Imperato21]. With some notable exceptions and considerable subtlety in subcategorizing the concept of trust in government, Sapienza and Falcone found that in reviewing 43 separate publications, trust in government correlated positively with vaccine acceptance, correlating with lower hospitalization rates and mortality [Reference Sapienza and Falcone22]. Similarly, trust in the healthcare system and science correlated to vaccine acceptance, with individuals who mistrust the healthcare system more susceptible to misinformation about the vaccines. The correlation of vaccine acceptance with trust in the healthcare system is significant since in the U.S. a 2023 Gallup poll showed that only 34% of the U.S. population had a high level of confidence in the medical system [Reference Saad23].
There are, however, complexities in interpreting the data from the COVID-19 experience, and perhaps missed opportunities. For example, a Pew poll in the early phase of the pandemic in April-May 2020 found an increase in the U.S. population’s trust in medical scientists to act in the best interests of the public, with an increase in the percentage of people answering “a great deal” from 2016 (24%) to 2020 (43%) [Reference Kennedy and Tyson24]. There was, however, a growing divide by political affiliation, with 32% of Republicans versus 37% of Democrats giving that response in 2016 and 27% of Republicans versus 52% of Democrats giving that response in 2020. By 2021, however, trust in medical scientists decreased to 29%, with a dramatic drop among Republicans (from 31% to 15%) and a more modest drop among Democrats (from 53% to 44%). The overall values among White, Black, and Hispanic groups were nearly identical (28% – 29%), with all groups demonstrating a downward trend [Reference Kennedy, Tyson and Funk25].
To increase trust in clinical research, first be worthy of trust
In the quest for increasing trust in clinical research, there must first be a demonstrable commitment to being worthy of trust and demonstrating that commitment in meaningful policies, leadership, and actions, as well as transparent collection of metrics assessing the implementation and impact of those policies and actions. The elements highlighted in Figure 2 provide a partial list of actions that will address serious concerns that have been expressed about the medical and medical research enterprise. Much has been written about how best to achieve this and it must be the foundation for all programs [Reference Griffith, Jaeger, Bergner, Stallings and Wilkins11,Reference Wilkins12]. A partial list of recommendations based on the above references and our own experience includes: 1. Establish strong, ongoing relationships with the community, emphasizing the institution’s commitment to the health of the community, and ensure that the clinical research workforce reflects the community’s diversity. 2. Conduct research at the highest levels of ethical and scientific quality. 3. Prioritize fairness, objectivity, openness, and respect for others, with programs that monitor research protocols and make adjustments as needed for compliance. 4. Emphasize the centrality of both initial and ongoing informed consent. 5. Acknowledge past unethical practices in clinical research and explain actions to prevent their recurrence. 6. Articulate institutional values and foster a culture of commitment to those values. 7. Communicate honestly and transparently. 8. Ensure the delivery of high-quality medical care in clinical research studies. 9. Emphasize consistency and continuity to principles that reflect an ongoing institutional commitment that transcends current leadership.
To increase trust in clinical research, expand the role of the clinical research nurse
To complement defining and measuring trust and trustworthiness [Reference Griffith, Jaeger, Bergner, Stallings and Wilkins11–Reference Stallings, Cunningham-Erves and Frazier13,Reference Cunningham-Erves, Villalta-Gil, Wallston, Boyer and Wilkins26,Reference Blendon and Benson27], and using that information to craft policies and programs to be viewed as trustworthy, we thought that there may be benefit in taking an orthogonal approach that focuses on identifying which professionals enjoy the most U.S. public trust. After analyzing why they enjoy that trust, one can then think about how best to incorporate those elements into the clinical research enterprise.
For the past 22 years, the nursing profession has ranked highest in the Gallup poll of Americans. In the 2023 poll, 78% of U.S. adults said nurses have high or very high honesty and ethical standards, with veterinarians (65%), engineers (60%), and dentists (59%) next in line, and all ranking above medical doctors (56%) and pharmacists (55%) [Reference Brenan28–Reference Brenan and Jones30]. Comparable figures for Members of Congress and U.S. Senators are 6% and 8%. In 2020, during the height of the pandemic, 89% of U.S. adults gave the highest marks to nurses. In light of the data on the impact of party affiliation on trust in medical scientists, the high rankings given to nurses in 2023 did not show much difference by political party (86% Democrats vs 76% Republicans), demonstrating that positive perceptions of nurses cut across political ideology. Rankings of medical doctors showed more of a partisan divide, with 73% of Democrats vs 54% of Republicans giving the highest ranking.
It is valuable, therefore, to consider what it is about nursing that accounts for this remarkably steady trust by the public, and potentially what can be learned that can be applied to the clinical research enterprise [Reference Kennedy and Tyson24]. There have been relatively few rigorous studies to identify the elements underlying public trust in nursing [Reference Girvin, Jackson and Hutchinson31], but there have been speculations on this topic [32], and so we offer for consideration several hypotheses based on a combination of public speculations about nursing in general and our own observations related to the role of nurses in clinical research. 1. By providing direct care to patients, nurses have the most patient contact time, including times when patients feel most vulnerable and frightened. 2. Nurses’ technical skills are evident to patients, as is the public’s knowledge of the intense training and rigorous professional certification of nurses. 3. Nurses are trained to consider the patient as a person rather than an illness, focusing on the ethical principles of “beneficence” and “respect for persons.” 4. Nurses often possess important communication skills, translating physician instructions in terms that may be easier for patients to understand, and making doctors aware of important changes in patients’ status. 5. Nurses rarely have competing agendas or priorities, and so they are very committed patient advocates, protecting their rights and making sure that their needs are met. 6. Nurses are usually both accessible and approachable. 7. The nursing staff generally reflects the demographic diversity of the community, so nurses are likely to be sensitive to cultural issues and capable of speaking in the research participant’s language. 8. The COVID-19 pandemic reinforced the public’s appreciation of nurses as selfless, front-line professionals who bravely cared for terribly ill patients despite the risks to themselves and their immediate families. This was especially true at the beginning of the pandemic when nurses enjoyed their highest ranking. The drop from 89% to 78% “high” and “very high” rankings for honesty and ethical standards from 2020 to 2023 may reflect problems related to nursing shortages that developed as a result of nurses leaving the workforce and perhaps the impact of several well-publicized nursing strikes.
Clinical research nurses play a vital role in the clinical research enterprise by ensuring that clinical protocols are followed faithfully and that adverse events are identified and documented. In addition, they play a vital role in the informed consent process, ensuring that participants understand their role both at the beginning and throughout the protocol, thus ensuring that the consent is ongoing. Moreover, research participants considering leaving a study may choose to discuss their concerns with the clinical research nurse, thus providing an opportunity for the nurse to convey this to the investigators who may be able to make adjustments to address the participant’s concerns. Importantly, the clinical research nurse has the expertise to understand the scientific rationale for the study, the hypothesis being tested, and the clinical implications of the interventions, while maintaining an independent patient-centered perspective. Thus, the clinical research nurse brings an extraordinarily wide range of important attributes and knowledge to the clinical research enterprise as a trusted professional who is in an excellent position to gain and retain the trust of participants [33,Reference Rutherford34].
Cataloging the elements that make up the unique role of the clinical research nurse allows one to consider how to maximize the nurse’s role in securing participant trust in the broader clinical research enterprise. We therefore offer specific suggestions to consider in strengthening the role of the clinical research nurse in the clinical research enterprise.
-
1. Engage clinical research nurses during the development of the protocol so that they understand the science and goals of the research, and so that they can actively participate as advocates for participants in optimizing the protocol design and assessing nursing feasibility. In addition, provide comprehensive training sessions at the beginning of the study and ongoing scientific education for the clinical research nurses as the protocol progresses through workshops, seminars, and progress meetings on the latest research findings. This will enhance nurses’ ability to address participants’ questions about the goals and design of the protocol and their concerns about potential risks and adverse events.
-
2. Ensure that a clinical research nurse reviews the concordance between the informed consent document and the protocol as written, and as participants are enrolled, reviews the elements of the consent and the protocol with the participants. Since the clinical research nurse will be responsible for coordinating the clinical activities of the protocol and caring for the participant, it is vital that the nurse concurs that the informed consent document matches the protocol requirements. After a participant is enrolled in the study, it is equally important that at their first meeting, the clinical research nurse reviews the details of the consent and the protocol with the participant so that the participant is fully prepared for what will happen during the protocol. During this crucial stage, the clinical research nurse can help demystify the study process for participants by providing clear, compassionate, explanations of protocol details and confirming the participant’s understanding. When necessary, they can also refer participants back to investigators to clarify aspects of the protocol and consent that the participant did not understand. This process reinforces the nurse’s importance and fosters a trusting relationship from the very outset of the study, providing comfort to the participant regarding safety and satisfaction, which are crucial for ongoing consent and can contribute to participant retention in the study.
-
3. Maximize continuity in clinical research nurse-participant interactions by ensuring that each protocol is assigned a lead clinical research nurse. For small studies, that nurse may be able to care for most or even all of the participants, but in larger studies, other nurses will also need to participate. Having a lead nurse promotes both continuity of care and consistency in the research process. It also promotes deeper personal connections and a better understanding of the participants’ unique needs and concerns. Regular check-ins with participants by the nurse, personalized follow-ups, and maintaining open lines of communication contribute to building trust and ensuring participants feel supported.
-
4. Maximize two-way communication between investigators and the lead clinical research nurse, including mechanisms for rapid communication if participants express concerns. Establish specific communication channels, such as regular meetings, digital platforms, and emergency contact systems, to facilitate prompt information sharing and collaborative problem-solving. Assigning a dedicated clinical research nurse to an investigator eliminates confusion about whom to contact, and thus saves time and provides the investigator ongoing access to clinical nursing expertise, which is crucial for maximizing clinical feasibility, and most importantly, research participant safety and satisfaction. When other nurses are required to help the lead nurse with a protocol, communication to the investigator from the nurses should go through the lead nurse to avoid a multiplicity of contacts and ensure changes are not introduced without the knowledge and agreement of the lead nurse. Thus, ensuring that nurses have a clear and direct line to investigators speeds the resolution of issues and helps maintain participant confidence in the study process.
-
5. Ensure that clinical research nurses conducting the study retain their primary identity as patient advocates. The trust that the public and research participants have in nurses derives in large part from the belief that the nurse is “there for them” without any other motive. Skepticism about medical researchers derives in part from concern that in their quest to answer a scientific question, they may not give as much attention as they should to the needs and desires of the participant. It is crucial, therefore, that enhanced participation by clinical research nurses in studies does not result in them being perceived as prioritizing the completion of the research over their primary independent responsibilities of focusing on participant vulnerability and providing direct nursing care, education, and support to the participants, their families, and their significant others. The nurse’s objectivity and disinterest are crucial to their being trusted by participants and thus need to be protected.
For all of the above reasons, individuals trained as clinical research nurses are also extremely valuable as members of the investigative and oversight teams as principal investigators, clinical research coordinators, nurse practitioner investigators, monitors, and auditors. These roles are separate, however, from the role of the clinical research nurse delivering the hands-on care and carrying out all of the activities specified in the protocol.
One logical extension of our analysis is to try to inculcate the elements of nursing that enhance trust into the training of all clinical research professionals. This logically starts with establishing the ethical principles that underlie clinical research, and then focusing on communication, cultural sensitivity, diversity, ongoing informed consent, participant advocacy, and mutual respect. Role playing may be an especially effective method for converting theory into knowledge [Reference Bharti35], with nurses providing feedback to trainees about their decisions in managing challenging situations. It needs to be emphasized, however, that nursing has a rich professional history and tradition of patient-centered, selfless service and nurses are a self-selected group of individuals who have made a commitment to nursing as a career. Other clinical research professional disciplines are newer and thus still establishing their values and traditions. They also commonly include individuals who plan to move into other fields. Moreover, since other clinical reseaerch professionals engaged in the protocol are committed to completing the research study, they may not be perceived as free of competing interests. Thus, there may be limits to capturing all of the elements that contribute to the trust the public has in nurses.
As the birthplace of American clinical research nursing under the visionary leadership of the legendary Nancy P. Ellicott, the first Superintendent [Reference Ellicot36], the Rockefeller University Hospital has a tradition of focusing on the vital role that research nursing plays in clinical investigation. This has included establishing the Heilbrunn Family Center for Clinical Research Nursing at Rockefeller [37], and advocating for the creation of the International Association of Clinical Research Nursing [38], the recognition of research nursing as a specialty practice, and the mastering of the fundamentals of research nursing by all nurses [Reference Eckardt, Hammer and Barton-Burke39]. Based on years of experience trying to optimize the research participant’s experience through the development of many programs [Reference Kost, Devine and Fernands40], we have adopted all five of the above practices.
The need for outcome measures of trust in clinical research
Assessing participant trust in clinical research is a crucial component of any program to enhance trust. We recognized that process measures, such as a signature or an informed consent form, do not provide actionable information. That is one of the reasons we embarked on a long-term project in 2006 to obtain outcome measures of participants’ experiences in clinical research. In collaboration with the National Institutes of Health (NIH) Clinical Center and 15 NIH-supported clinical research institutions, including many Clinical and Translational Science Award (CTSA) sites, we developed a specially designed Research Participant Perception Survey (RPPS) based on feedback from a diverse cohort of participants and then validated it with data from almost 5,000 participants [Reference Kost, Lee and Yessis14,Reference Kost, Lee, Yessis, Coller and Henderson41,Reference Yessis, Kost, Lee, Coller and Henderson42]. The RPPS has evolved by a rigorous process to make it shorter, thus requiring less time to complete, and easier to deploy by individual research sites [Reference Kost and de Rosa43,Reference Kost, Cheng and Andrews44]. In total, a version of the RPPS has been deployed to more than 50,000 participants at more than 25 research organizations since its creation.
In the original RPPS, we asked “Did you have trust and confidence in the research doctor/investigator?” and “Did you have confidence and trust in the nurse/coordinator?,” for which responses were remarkably similar. In the latest version of the RPPS, we asked “Do you have confidence and trust in the study team?” We have carefully tracked responses to the latter question because they correlate best with participants’ overall assessments of their research experience. Over the years since 2013, positive scores for these questions from responders to the survey have ranged from 86% to 95% at Rockefeller.
One of the things we learned from the early RPPS studies was that participants were almost universally motivated by altruism, often mixed with other motives, such as the perceived importance of the topic and compensation and anticipated benefit (conditional altruism) [Reference McCann, Campbell and Entwistle45], and that they wanted to be valued as partners in research. Based on this, combined with findings that only two-thirds of our participants knew how much we valued their participation, we initiated a participant appreciation campaign that initially included visible banners, brochures, and participant appreciation lapel pins, and then was incorporated into the values we taught in our clinical research training program. Over the ensuing 10 years we observed a positive trend in the high ratings of the response to the question, “Did you feel like a valued partner in the research process?,” which rose from approximately 63% to approximately 90% of survey respondents answering “always.” (unpublished data). Thus, we believe that obtaining outcome data that can lead to specific interventions is crucial for making progress in building trust and confidence in the clinical research enterprise.
One special case worthy of analysis is studies in which the principal investigator is a nurse. Since 2014, as part of program of the Heilbrunn Family Center for Clinical Research Nursing, we have been funding fellowship awards of up to $25,000 to support clinical research protocols developed by PhD candidates and postdoctoral nurses at sites around the U.S. To date, we have funded 45 Heilbrunn Nurse Scholars. We now have a sufficient cohort to try to assess with the RPPS whether nurse-led studies differ in participant scores related to trust from studies led by individuals other than nurses.
In conclusion, trust is a vital and necessary component in clinical research since the latter invariably requires that a participant accept the risk of turning over control of an aspect of her or his life to the investigative team. That leap of faith made by participants requires that they believe in the competence, knowledge, and integrity of the researchers, and that they feel that their values and the investigator’s values are aligned. Many factors affect the attitudes and beliefs of populations, subgroups, and individuals, and these add complexity to fully understanding the factors that sum into a person having sufficient trust to enter a clinical study. That confidence is not easily won, and it can be lost very rapidly, so it needs to be the centerpiece of the clinical research enterprise. The COVID-19 pandemic has stimulated a dramatic increase in research on trust, but there remain many open conceptual, theoretical, methodological, and analytical questions, leading to considerable confusion and uncertainty about the best way forward in rebuilding public trust, especially among an increasingly politically polarized society. In the interim, we suggest that one way forward is to learn from the high esteem in which nurses are viewed across society, including across the political spectrum, and enhance their role in the research enterprise and, perhaps by extension, in public health messaging.
Author contributions
BSC: Conception and design, critical intellectual contribution, initial and final draft of manuscript. RD, CC, RK, and JK: Critical intellectual contribution and contribution to drafts of manuscript.
Funding statement
Supported in part by grant UL1 TR001866 from the National Center for Advancing Translational Sciences, NIH CTSA program.
Competing interests
The authors have no conflicts of interest to declare.




