Introduction
Launching multicenter clinical trials is complex and time-consuming. Because review and negotiation of research-related agreements is a primary cause of delays in trial startup, improving this process allows for an opportunity to make significant progress in improving clinical trial efficiency [Reference Lai, Forney, Brinton and Simpson1,Reference Dilts and Sandler2]. Regardless of funding source, new studies may require the review and execution of several agreements (e.g., confidential disclosure agreement, clinical trial agreement). Estimates of the cost due to delays in contracting for a pharmaceutical trial sponsor range from $600,000 to $8 million per day of delay including the cost of lost sales of a potential drug [3]. Additionally, lags in startups have a significant human cost, delaying the evaluation and prescribing of potential new treatments [4]. Data from the 2010 Clinical and Translational Science Awards (CTSA) Contracts Processing Study revealed that negotiating contract terms for a clinical trial agreement required a mean time of 55 days, but that use of a master agreement could reduce the mean for full negotiation to 22 days [Reference Kiriakis, Gaich and Johnston5]. A master clinical trial agreement (“Master Agreement”) is a formal agreement made in advance between an industry sponsor and a site that establishes core terms for clinical trials initiated between the parties. Each study under the Master Agreement can be initiated with a simple study-specific letter acknowledging the Master Agreement and identifying the study-specific budget and protocol without requiring any negotiation of legal terms between the parties.
Why Is a Sponsor Master Agreement Not Enough?
Sponsor-specific Master Agreements continue to be used, despite limited data to illustrate their level of uptake and efficacy. Industry sponsors have unique Master Agreements that are pre-negotiated with each of their major partner sites. Negotiating a sponsor Master Agreement can take many months, or in some cases, years, and the agreements may have limited effective periods of 3–5 years [6]. A sponsor-specific master agreement can be very effective but has drawbacks associated with being costly and time consuming at startup. The ACTA can add value in situations where the cost and effort to craft a Master are not worth it to the involved parties or in cases where resources may be limited.
Leading Master Agreement Innovations: The CTSA Program and the Trial Innovation Network
Significant national efforts have been made to address issues around research-related agreements [7,8]. The CTSA Program and the Trial Innovation Network (TIN), supported by the National Center for Advancing Translational Science (NCATS), aim to transform biomedical research processes to increase the speed by which discoveries are translated into practice [Reference Califf and Berglund9–Reference Bernard, Harris and Pulley11]. The TIN is a collaborative initiative within the CTSA Program and is comprised of three key partners – the > 60 CTSAs, the Trial Innovation Centers (TICs), and the Recruitment Innovation Center [Reference Wilkins, Edwards and Stroud10,Reference Bernard, Harris and Pulley11]. As part of their changes to address scientific and methodological challenges, the CTSA Program and TIN were charged with overcoming administrative and institutional organizational barriers [Reference Califf and Berglund9]. Tackling issues with contracting delays became part of the CTSA mission in 2012 when NCATS and the CTSA Program launched the Accelerated Research Agreements (ARA) Initiative, whose mission is to provide agreements that are acceptable to participating institutions and organizations in an effort to expedite the study initiation process [12]. This initiative was furthered in 2016 with the launch of the TIN Standard Agreements workgroup.
The ARA Initiative and the Standard Agreements workgroup sought to promote a model for creating standard agreement templates that most institutions and many sponsors could accept, thereby negating the need for duplicative and time-consuming negotiations. This communication describes efforts to develop and refine solutions and focuses on the two most heavily used standard agreements stemming from these initiatives, the Accelerated Clinical Trial Agreement (ACTA) and the Federal Demonstration Partnership Clinical Trial Subaward Agreement (FDP-CTSA).
Developing and Refining a Solution: The ACTA
Industry funds nearly six-fold more clinical trials than federal sources and accounts for roughly 70% of dollars spent on clinical drug trials [Reference Ehrhardt, Appel and Meinert13,Reference Beal, Dean, Chen, Dragaon, Saulino and Collard14]. The ACTA focused on multisite, industry-sponsored trials and was the first template to come from the ARA Initiative. The goal of the ACTA was to create a straightforward document that clearly sets forth the contractual obligations of both parties in language that includes pre-agreed-upon compromises to contract terms. The ACTA was developed by a workgroup comprised of legal and contracting experts from 25 major academic institutions and medical centers, engaged in clinical and translational research, in collaboration with the University-Industry Demonstration Partnership (UIDP) [15] and with input from several pharmaceutical companies (Workgroup members: https://ara4us.org/acta/work-group-membership/).
The ACTA differed from other concurrent standard agreement efforts, including MAGI (Model Agreements & Guidelines International) and TransCelerate, in that (1) it focused on creating a full draft contract rather than a term library; (2) the final agreement was based on terms that both parties usually end up agreeing to after multiple rounds of negotiation (i.e., compromised approach); (3) the agreement was created with support from both industry and academic partners; and (4) this effort received significant NIH support, as well as organized outreach and promotion [16].
In brief, the ACTA was developed by creating a list of 28 terms specific to clinical trial contracting. The terms were sent to workgroup members to prioritize and rank from least to most difficult to negotiate. The workgroup defined the common position of academic institutions and industry for each of the most challenging terms. Using the preferred positions of each party as the starting point, the workgroup proceeded to craft compromise language for the terms being included in the ACTA standard agreement. The most difficult to negotiate were Insurance, Confidential Information, Publication, Limitation of Liability, Subject Injury, Data Use/Ownership, Intellectual Property, and Indemnification (See Table 1). To arrive at final terms, the workgroup utilized contract language that was often the final position arrived upon in previous contracts after negotiation – i.e., starting with the most frequent compromise position. For more challenging terms, industry and academic partners shared their concerns related to risks and local context/laws to construct language that addressed most concerns even if it could not completely mitigate all risks. Industry sponsors, organizations, and academic institutions reviewed final draft language with extensive discussions to arrive at the final agreement with terms that were acceptable, if not completely satisfactory, to all parties.
Table 1. Controversial terms negotiated for the Accelerated Clinical Trial Agreement (ACTA)
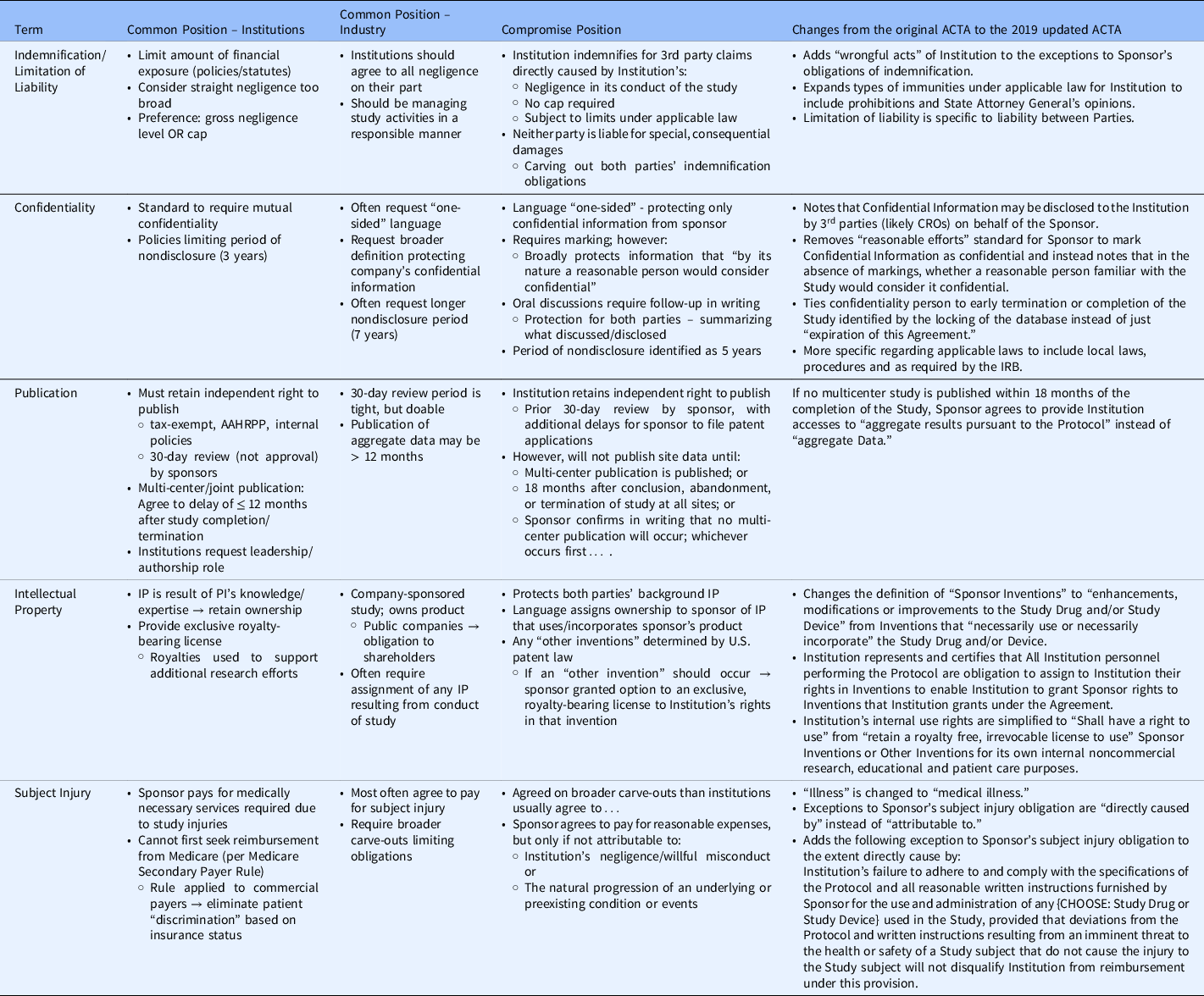
Following term harmonization and development of the ACTA, the standard agreement was reviewed by a larger workgroup with representatives from each of the > 60 CTSAs and piloted with five studies from five unique sponsors across ∼ 40 sites to look for serious flaws in the document. There were no substantive changes and after several months of socializing the terms through meetings with the CTSAs, the ACTA was published in October 2014.
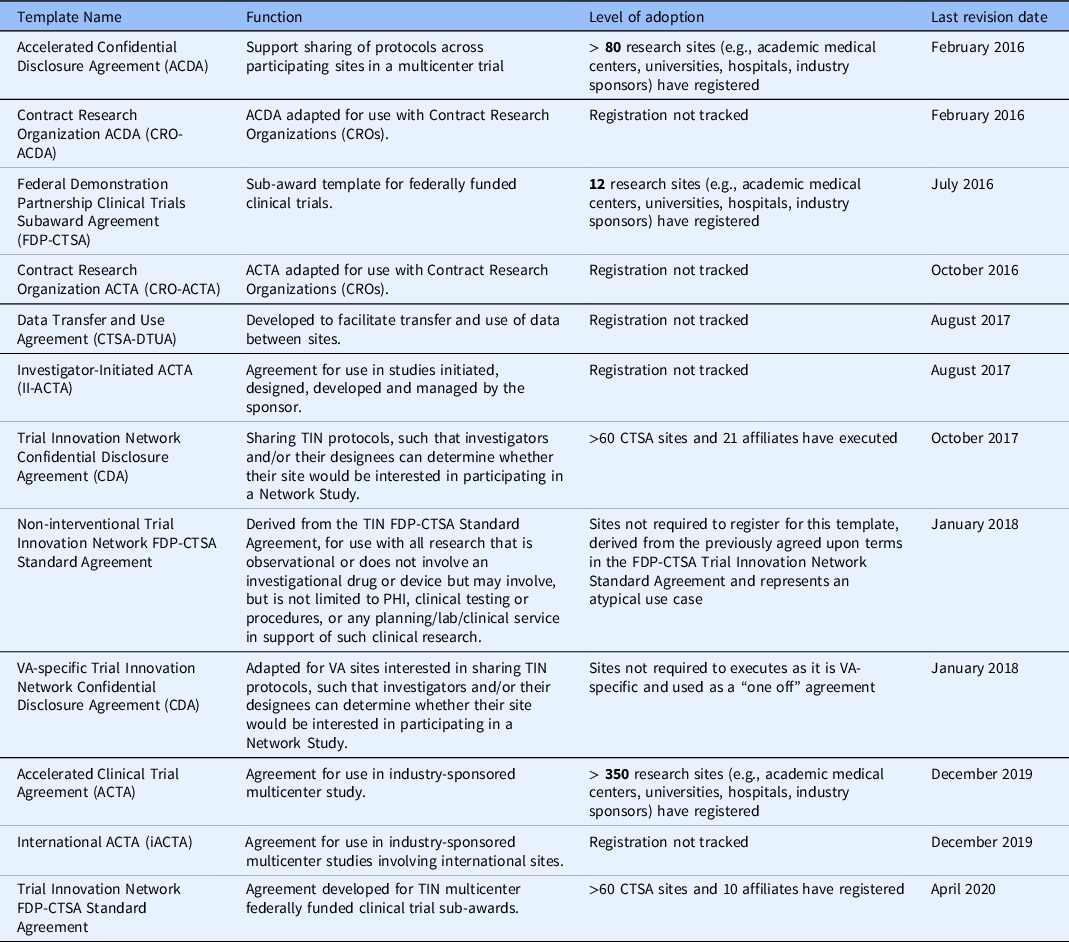
The ACTA continues to be refined by a small workgroup composed of many of the original ACTA workgroup members to keep pace with changes in federal guidelines and the new methodologies being used to conduct clinical trials (e.g., remote monitoring and electronic consenting). From the genesis of the ACTA in its initial iteration to its current version, the terms have evolved to address existing or emerging issues from the sponsors and sites (e.g., updated record retention language inclusion of CRO and affiliate assignment language) and to include new language that reflects the changes in the conduct of clinical trials (e.g., data security and cloud-based portals to house participant data, the Sunshine Act [17], Human Research Protection Program Accreditation standards). The newest version of the ACTA is also more clearly delineated to be used with Phase II studies and beyond. After establishing a model for creating the ACTA template, additional agreements were developed (See Table 2) to address other common scenarios in clinical trial conduct that require specific agreements, such as investigator-initiated trials and confidential disclosure agreements. Sites can register to use any or all the template agreements as appropriate to their institution’s policies and needs.
Table 2. Agreements developed by the CTSA/Trial Innovation Network as part of the accelerated research agreements initiative
Quantifying the Impact of Standard Agreements
The support and CTSA resources allocated to developing standard agreements led to the prioritization of capturing metrics of usage and potential time savings for the various template agreements. However, there were several challenges in collecting metrics due to a lack of harmonization for contracting data elements. Specifically, data points such as T0 (the initial time identifying the start of the contracting process) and TFINAL for contract negotiations are often defined differently across centers. Recognizing these limitations, the group also focused its data collection on standard agreement usage rates along with assessing what materials and methods existed at various sites to promote and educate on standard agreements.
CTSA usage of ACTA
The ACTA workgroup developed a REDCap-based survey to assess usage and potential time savings across the CTSA and other institutions associated with the ARA Initiative [Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde18]. Survey elements included use of the ACTA and average calendar days from the date the package (defined as all necessary documents needed to begin the process) was received to the date all terms were negotiated (defined as terms complete but may be waiting on IRB or budget) for the ACTA as compared with nonstandard agreement templates. Two separate rounds of survey data were collected. The first round of data was collected during a 24-month period from 2015 to 2017. The survey was sent to the points of contact for all CTSAs and organizations who supported the original development and review of the ACTA as part of the larger ACTA workgroup. There were 55 organizations and CTSA institutions that responded and reported that the ACTA was used ∼ 90 times with an average time savings for full negotiation of 40 days. Contracting processes are highly variable in nature given the range of factors that can impact their full execution, and estimates collected in the survey reflected this variability, ranging from seven days to six months. The total number of studies conducted across more than 55 organizations and institutions was not captured; therefore, we are not able to provide a denominator for 2015–2017 use.
In 2021, a second survey was sent to 13 CTSA sites active in the smaller ACTA workgroup that helped draft the original ACTA and who completed the initial round 1 survey, requesting information on use of standard agreements negotiated during 2019–2021, with specific emphasis on negotiation time in days for the ACTA versus no standard agreement (See Fig. 1). The follow-up survey explored whether ACTA usage had increased over time. Seven organizations responded with complete data sets for ACTA usage and negotiation times with and without the ACTA or standard agreement. The ACTA was used 122 times across the seven sites and demonstrated an average time savings for full negotiation of 55 days when compared to negotiations without the ACTA or standard agreement.

Figure 1. Negotiation time of agreement (excludes budget negotiation).
Note: * Negotiation time less than one day; ¥ This site included sponsor review time in its metrics which accounts for a significant portion of the negotiation time.
To date, > 350 research sites, including academic medical centers, universities, hospitals, physician practices, and industry sponsors, have agreed to the terms of the ACTA and to accept it without revision. In addition, the ACTA agreement has been downloaded>1,500 unique times from ara4us.org.
Adapting This Solution for Federally Funded Clinical Trials: The FDP-CTSA
With the industry-focused ACTA work progressing, NCATS, CTSA leadership, and the FDP pivoted to address the nuances associated with a federal sub-award agreement template for clinical trials. In June 2015, a new workgroup comprised of representatives from a subgroup of CTSAs, the original ACTA workgroup, and FDP member institutions was convened to focus on drafting a standard federal sub-award agreement [19]. The sub-award template was premised upon: use of an NIH Sponsor, Fixed Price, domestic enrolling sites, compliance with all federal regulations, adherence to ACTA terms when possible, and allowance for the addition of study-specific terms. The resulting standard agreement, the FDP-CTSA, was officially approved for use by the CTSA stakeholders, the NIH, and the FDP in August 2016 and updated again in 2020 when the FDP amended its template to reflect changes in federal guidelines [20].
National adoption of the FDP-CTSA was furthered in 2016 when the TIN was launched. As part of its innovations, the TIN adapted the FDP-CTSA for use by the network and its standard agreements initiative to accelerate study startup across CTSA sites and their affiliates. The TIN workgroup used the FDP-CTSA as a starting point and made edits to terms within Attachment 2B (as allowed by the FDP) to better align with the needs of TIN studies. Attachment 2B contains study or network-specific special terms and conditions. This template is officially known as the FDP-CTSA Trial Innovation Network Standard Agreement (“TIN Standard Agreement”).
Once completed, the workgroup sent the TIN Standard Agreement to all CTSAs in the network and requested that each site register its acceptance of the standard agreement with the expectation that use of the agreement would reduce negotiation delays and allow for fast tracking of review once an actual study was issued. Only 2 CTSA sites did not register to use the TIN Standard Agreement. Ten CTSA affiliates also registered to use the agreement indicating their willingness to use this document as a starting point for contract negotiations in TIN studies.
TIN Usage of FDP-CTSA
The TIN gathered standard agreement metrics in those circumstances when the TIN FDP-CTSA was used in a multisite trial with a TIC (i.e., Duke, Utah) serving as the coordinating center (See Fig. 2). The use of a designated coordinating center allowed for control of more variables in the contracting process, including consistency in defining which standard agreements would be used for contracting.

Figure 2. TIN studies that have utilized standard agreement services (i.e., TIN FDP-CTSA) wherein a TIC site acted as the study coordinating center. Days refer strictly to the contracting process and do not relate to IRB review or budget negotiations. Median days for negotiation. CMV ValEAR = 41; DOSE = 46; SPIRRIT = 151; STRESS = 64; TRANSFORM-HF = 46; SILDI-SAFE = 109.
Six multicenter studies were identified who were awarded (and utilized) Standard Agreement services wherein a TIC acted as the study coordinating center and facilitated the contracting process using the TIN FDP-CTSA Standard Agreement (See Fig. 2). There was a clear dichotomy across the studies with three studies having median days for negotiation at<46 days (median = 41, 46, 46), and the remaining three studies taking 64 days to well over 100 days for median negotiation time. Delays in negotiation were generally related to nuances of the associated studies rather than requests to change template language including one study being the first TIN study to use the new TIN FDP-CTSA (median = 64), another study had issues with the Study Drug term as several sites had concerns with the version of drug being provided by the industry sponsor (median = 109), and one study protocol was written by an EX-US sponsor which led to a flow down of General Data Protection Regulation (GDPR – regulation in European Union for protection and privacy) that was a required as a separate attachment to the agreement (median = 151).
Standard Agreements and TIN-Supported COVID-19 Studies
Four COVID-19 studies received TIN support and illustrate recent use cases for multisite studies using a standard agreement template: ACTIV-1 (NCT04593940), ACTIV-4 Host Tissue/Novel Experimental COVID Therapies Affecting Host Response (ACTIV-4HT/NECTAR - NCT04924660), ACTIV-6 (NCT05736861), and Passive Immunity for Our Nation (PassItOn - NCT04362176). The smallest study had 28 sites (accrual goal of 1,000 participants) and the largest study had 74 sites (accrual goal of 15,000 participants). By using a standard agreement (e.g., FDP-CTSA or similar) the studies demonstrated significant time savings in average days to full contract execution across all study sites as compared to historical data. ACTIV-4HT/NECTAR had the longest average time to full contract execution with 75 days versus 103 days from historical data, and PassItOn had the shortest average time at 16 days [Reference Kiriakis, Gaich and Johnston5]. This equates to time savings of 27 to 87 days which exceeds what would be expected for full contract execution with a non-master agreement, even when taking into consideration that some of this time savings is attributable to the prioritization of COVID-19 trials (which included putting other non-COVID studies on hold).
Lessons Learned
Challenges, Limitations, and Unanticipated Outcomes
Despite challenges to obtaining quality data on standard agreement usage, efforts have been made to assess usage of the ACTA and FDP-CTSA. Notably, our findings from the 2015-2017 CTSA survey of ACTA usage align with results from the University of California, Biomedical Research, Acceleration, Integration, and Development (UC BRAID) study, in which use of standard agreements was found to reduce negotiation time to an average of 39 days as compared to 73 days without a standard agreement [Reference Tran, Bowman-Carpio and Buscher21].
In addition to limitations with metrics collection, it should be noted that there are several potential confounders that exist within our standard agreements data including size of the institution and contracting office, number of studies conducted at a site per year, and inclusion of international sites among others. Future work should be done to understand how these variables impact the standard agreement process and negotiation time.
An unanticipated outcome of the ARA initiative is the use of modified standard agreements. While the intent of the initiative was for sites to use the standard agreements in their unmodified form, many sites have found success with using the ARA templates as starting points for negotiations. For example, some sites may be unable to use all the ACTA terms, so they use a modified ACTA (aka “MACTA”) in which negotiations are focused on a few specific terms rather than the whole agreement. Section 8, “Inventions, Discoveries, and Patents” is the section most often modified by ACTA users and is the one that requires the most review when Phase I studies seek to use the agreement. To avoid confusion, sites using the MACTA are asked to remove the ACTA moniker as that term is exclusively for the unmodified version.
There is anecdotal evidence of time savings when using the MACTA, however, it is difficult to measure as many sites do not routinely track use of the MACTA. MACTA data collected from Mayo Clinic and the University of Utah between 2019 and 2021 shows that the MACTA was used 36 times at Mayo for an average agreement turnaround time of 28 days and 30 times at the University of Utah for an average turnaround time of 58 days.
Dissemination
Dissemination and education are key to supporting and streamlining standard agreements. The virtual home for the ARA initiative is the ara4us.org website, where interested research organizations can obtain more information about the initiative and follow its progress [12]. The website allows any organization to download the latest versions of the ARA initiative’s templates, register to use individual agreements, see partnering sites, and identify workgroup members who contributed to the development of each agreement [12]. Registering for an agreement indicates that the registering institution would be willing to use that agreement and that the institution can be listed as an ARA participating organization on the website. Registration does not obligate an entity to use the standard agreement but rather allows for institutions and sponsors to view the website to determine potential parties to participate in upcoming studies and identify points of contact for outreach.
The ARA initiative has conducted outreach to research professionals and administrators, including MAGI, National Council of University Research Administrators, and Society of Research Administrators, to raise awareness regarding the ACTA, FDP-CTSA, and other standard agreements. Additional education around the ARA initiative, the template agreements, and sites using them, is encouraged at the local level for onboarding new staff. Some sites have created processes to move those studies using standard agreements to the front of the contracting queue to promote adoption.
Examples of Best Practices
A study of contract processes across Mayo Clinic sites in Minnesota, Florida, and Arizona provided further insight into strategies to help streamline contracting. In addition to utilizing the ACTA, the centers created methods to eliminate silos between business units, thereby allowing parallel processes such as IRB review, contracting, and budgeting to occur and reduce delays [Reference Watters, Pitzen and Sanders22]. The group also found that assigning a trained project manager to facilitate each trial through the activation processes (i.e., contract, protocol, budget, IRB) led to the greatest reduction in startup delays [Reference Watters, Pitzen and Sanders22].
The UC BRAID standard agreement study echoed some of the best practices identified by Mayo, specifically engaging in parallel processes and breaking down silos between business units. The group went further to say this could be accompanied by systematic and transparent collection of key metrics related to each step of site activation [Reference Tran, Bowman-Carpio and Buscher21]. The group also suggested the need for developing shared technology such as databases or platforms for storing key metrics and clinical trial information that could be accessed across departments, thereby reducing redundant efforts and promoting parallel processes [Reference Tran, Bowman-Carpio and Buscher21].
Taken as a whole, these best practices suggest that successful optimal implementation of standard agreement templates and faster study activation requires cross-departmental institutional commitment. Centers must make a business decision to support collaborative streamlining efforts across relevant departments. The ACTA provides an excellent framework and centerpiece for coordinating these efforts.
Conclusions
Mounting evidence in the literature and across the CTSA Program demonstrates time and labor savings from use of standard agreement templates. The CTSA is in a unique position to help motivate and mobilize contracting offices across the consortium to gather common metrics associated with the contracting process and to help determine and share best practices. Future directions for this work should include developing harmonized contracting metrics, gathering data from across the CTSA, continuing education and outreach efforts, embedding best practices in standard agreements usage within various groups, and then assessing impact. Additionally, ever-changing regulations (e.g., newly required telecommunications language) and the impact of the pandemic on the way clinical trials are conducted and monitored will necessitate review and updating of the ARA initiative’s standard agreement templates.
Acknowledgments
The authors extend their sincere thanks to all the members of the ACTA large and small workgroup as well as Trial Innovation Center standard agreement experts for their contributions to developing and supporting the template documents, as well as in metrics collection.
Funding statement
This project was supported by awards: VUMC CTSA (UL1 TR002243), and the Trial Innovation Centers (U24TR001608, U24TR001597, and U24TR001609) from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Competing interests
The authors have no conflicts of interest to declare.






