Introduction
The National Institutes of Health seeks to “improve health, revolutionize science, and serve society,” and the National Center for Advancing Translational Sciences supports translational research so new treatments “reach people faster” [1,2]. These objectives emphasize broader impacts of research that may influence areas such as economy, public policy, or society [Reference Penfield, Baker, Scoble and Wykes3]. Systematically evaluating research impact is especially important in T2 translational research to establish intervention effectiveness and clinical guidelines, which can inform T3 implementation research [Reference Fort, Herr, Shaw, Gutzman and Starren4]. Due to methodological challenges, organizational inertia, and resource constraints, academic outputs of T2 studies may not be as robust as those from basic science or T1 studies [Reference Woolf5]. However, understanding and evaluating impact beyond academia is essential to supporting the value proposition of T2 research.
The Translational Science Benefits Model (TSBM) is a framework designed to identify and define areas of clinical and translational science that provide benefits to public health and society [Reference Luke, Sarli and Suiter6]. The TSBM highlights potential benefits that result from scientific innovations and stresses pathways and mechanisms through which these potential benefits can be realized. The aim of the TSBM is to bridge the gap between research discoveries and practical application by understanding that translational research has value beyond traditional quantitative measures used to assess research value [Reference Luke, Sarli and Suiter6]. The TSBM provides a structured approach by guiding researchers, policymakers, and stakeholders in making informed, evidence-based decisions about how to best apply scientific findings in routine settings.
The QUARTET USA trial was a double-blinded, randomized trial conducted at Access Community Health Network (ACCESS), a federally qualified health center network (FQHC) in Chicago, USA. The aim of QUARTET USA was to evaluate whether ultra-low dose quadruple combination therapy lowers blood pressure more effectively compared to standard dose monotherapy in patients with hypertension. The purpose of this report is to evaluate the impact of the QUARTET USA trial, a T2 stage translation trial that focused on evaluating effectiveness and safety of an intervention in a controlled setting, using the TSBM.
Methods
The methods and results of the QUARTET USA trial have been published [Reference Baldridge, Huffman and Lazar7,Reference Huffman, Baldridge and Lazar8]. Briefly, the trial used a type I hybrid effectiveness-implementation, phase II randomized (1:1), double-blind design to evaluate efficacy and safety of an ultra-low (i.e., quarter standard) dose combination of four blood pressure lowering medications among patients with hypertension who receive care at ACCESS. Participants were recruited and randomized from August 2019 to May 2022. Participants received either a (a) quadpill of candesartan 2 mg, amlodipine 1.25 mg, indapamide 0.625 mg, and bisoprolol 2.5 mg or (b) candesartan 8 mg for 12 weeks. Participants in both groups had open-label amlodipine 5 mg daily added to their regimen at 6 weeks post-randomization if their blood pressure was ≥130/≥80 mm Hg. The primary outcome was between arm difference in systolic blood pressure (SBP) change at 12 weeks. Selected secondary outcomes included between arm difference in diastolic blood pressure (DBP) change at 12 weeks and proportion of add-on amlodipine. Safety and tolerability were also assessed. The primary outcome was assessed in a modified intention to treat population using a linear mixed model with fixed study arm, study visit, and baseline outcome value effects and a random participant effect to account for within-participant correlation. We used a two-sided p < 0.05 to define statistical significance without adjustment for multiple testing.
This examination of the translational impact of the QUARTET USA trial was conducted using the Translating for Impact Toolkit Case Study and Impact Profile tools from October 2023 to April 2024. The first and last author (GI, MDH) completed the case study and impact profile and sought review and edits from the other authors. This examination followed a guided process where processes and outcomes from the trial were assessed for impact across the 30 potential benefits and 4 major domains (i.e., Community and Public Health, Clinical, Economic, and Policy) outlined by the TSBM [Reference Luke, Sarli and Suiter6]. The distinction between processes and outcomes was also done to differentiate between study actions undertaken in pursuit of the trial versus study outcomes discovered because of the trial. For example, training of research staff and establishing manufacturing partnerships would be considered a process as these are done to enable the research. Results that evaluate hypotheses of primary, secondary, or safety outcomes would be considered outcomes as they are a result of the conducted research. Both processes and outcomes have the potential to generate impact as defined by the TSBM.
Results
Summary of QUARTET USA results and process evaluation
Among the 62 randomized participants (n = 32 intervention, n = 30 control), mean (SD) age was 52 (11.5) years, 45% were female, 73% self-identified as Hispanic, and 18% self-identified as Black. Baseline mean (SD) SBP was 138.1 (11.2) mm Hg, and baseline mean (SD) DBP was 84.3 (10.5) mm Hg. There was no significant difference in SBP change between the intervention and control arms at 12 weeks (–4.8 mm Hg [95% CI: –10.8, 1.3, p = 0.123]). However, there was a significant difference in DBP between the arms (−4.9 mmHg [95% CI: –8.6, –1.3, p = 0.009]). Add-on amlodipine rate at 6 weeks was lower in the intervention arm (19% vs 53%, model estimated Odds Ratio = 0.08 [95% CI: 0.02, 0.41], p = 0.003). Adverse event rates were similar between study arms [Reference Huffman, Baldridge and Lazar8].
In addition to the primary study results, the QUARTET USA process evaluation found that among the 26 (out of 32, 81%) participants in the intervention arm who completed post-trial surveys, 80% were satisfied with combination therapy, 96% reported that benefits of combination therapy outweighed the risks and that it was convenient to take [Reference Sanuade, Jacobson and Quintana9]. Qualitative analysis also showed that both participants and healthcare professionals believed combination therapy reduced perceived pill burden and encouraged medication adherence [Reference Sanuade, Jacobson and Quintana9].
Measuring impact using the translational science benefits model
Figure 1 outlines the benefits from the QUARTET USA trial. There were eight benefits identified in the Clinical and Medical (5 benefits), Community and Public Health (2 benefits), and Economic (1 benefit) domains. There were five benefits in process measures and two benefits in terms of outcomes.
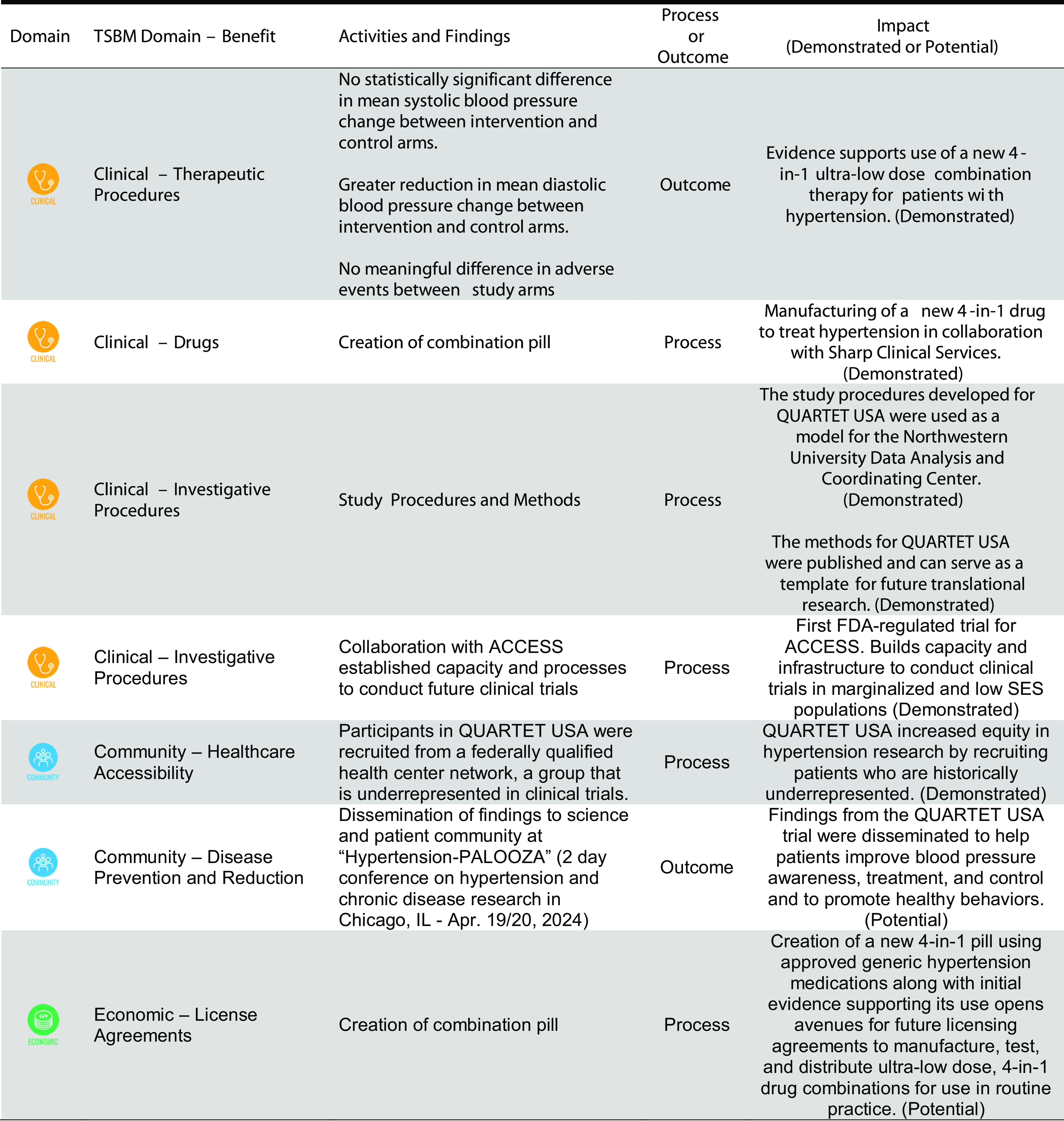
Figure 1. Impact from QUARTET USA trial based on domains outlined by the translational science benefits model (TSBM). ACCESS = access community health network; FDA = food and drug administration.
The impacts demonstrated in the Clinical and Medical domain include contributing evidence for the use of ultra-low dose quadruple combination therapy for patients with hypertension (Therapeutic Procedures), the manufacturing of a new 4-in-1 drug combination (Drugs), development of study procedures for data coordination and trial methods (Investigative Procedures), and capacity for Food and Drug Administration regulated trials at ACCESS(Investigative Procedures).
The impacts demonstrated in the Community and Public Health domain include addressing equity in hypertension research by including participants who have been historically underrepresented in research (Involvement in Clinical Trials). The impacts also included dissemination of findings through a science and community dissemination meeting to improve blood pressure awareness, treatment, and control through pharmacotherapy and promote healthy behaviors (Disease Prevention and Reduction [Potential]).
The potential impact in the Economic domain related to evidence created through the QUARTET USA trial that may support future licensing agreements to manufacture, test, and distribute ultra-low dose, 4-in-1 drug combinations for use in routine practice.
Discussion
Using the TSBM, 8 benefits of impact across 3 domains were identified from the QUARTET USA trial. This evaluation provides the primary, secondary, and safety outcomes reported in the primary study report, as well as the results from the trial’s process evaluation [Reference Sanuade, Jacobson and Quintana9].
While the QUARTET USA study failed to reject the null hypothesis for its primary outcome, the direction and magnitude of effect were similar to the related QUARTET trial conducted in Australia, which suggested a –6.9/–5.8 mm Hg average greater SBP/DBP lowering effect at 12 weeks [Reference Chow, Atkins and Hillis10]. A study-level meta-analysis of four trials (n = 779 participants), including QUARTET and QUARTET USA, supports the blood pressure-lowering effects of ultra-low dose quadruple combination therapy [Reference Bennett, Chow and Chou11]. An individual-level pooled analysis of these three trials is ongoing, which supports the impact wherein the trial contributes to the overall body of evidence related to ultra-low dose quadruple combination therapy.
Most benefits from QUARTET USA were related to the research process, whether through drug manufacturing, trial procedure, conduct and analysis, or inclusion of participants from groups who are historically underrepresented in research. Two benefits are potential and thus should be considered as such. The development of a fixed, ultra-low dose quadruple pill is impactful because it directly addresses low medication adherence and is often able to reduce the need to up-titrate medications [Reference Sanuade, Jacobson and Quintana9,Reference Ho, Bryson and Rumsfeld12]. These are often mentioned as driving factors for low hypertension control rates. The potential for a licensing agreement to develop this medication is very important because it allows for scalable and sustainable production of the medication independent of grant funding this treatment protocol should be shown to be effective.
The evaluation of research impact is increasing but remains limited. The strengths of the TSBM are its comprehensive yet flexible nature and inclusion of multiple tools to plan, assess, and disseminate impact. On the other hand, this assessment also has potential limitations. First, the TSBM is not the only research impact framework, and it is possible that others such as the Framework to Assess the Impact of Translation health research (FAIT) or the Research Excellence Framework (REF) may provide a better assessment, or at the very least offer a different perspective on assessing impact [Reference Dodd, Ramanathan and Angell13,Reference Sutton14]. We selected the TSBM based on our familiarity with the tool and availability of support from Washington University Institute for Clinical and Translational Sciences to use this tool. Second, there is also the possibility that some study impacts do not fit neatly into one of the 30 current benefits identified in the TSBM. For example, the QUARTET USA trial built capacity for FDA-regulated clinical trials at ACCESS through training of research staff as well as support for early-stage investigators. It also allowed ACCESS to build its larger research infrastructure through the use of its electronic health record’s patient portal and SMS texting capacity to reach out to patients who may qualify for studies. These efforts support and enable future participation in clinical trials and healthy equity. Third, the assessment was conducted post hoc after trial completion by members of the trial team, which may lead to recall bias or ascertainment bias. However, intimate knowledge of the trial and its process and outcomes is necessary to evaluate impact. An independent assessor may be a more reliable approach than using study team members to carry out these assessments, though no such comparison in evaluating the reliability of impact ascertainment has yet been conducted to our knowledge. Fourth, some benefits may occur in the future, and while we sought to identify those a priori as “potential” benefits, there may be others that we have not anticipated.
In conclusion, we assessed and reported the impact of the QUARTET USA trial using the TSBM, which showed eight benefits across Clinical and Medical, Community and Public Health, and Economic domains. Future research is needed to systematically and prospectively evaluate research impact to help disseminate and translate research into routine practice.
Acknowledgements
We acknowledge the patients and teams at ACCESS. We thank members of the QUARTET USA investigators and team, including Dr Hiba Abbas, Ms. Fallon Flowers, Ms. Adriana Quintana, Ms. Alema Jackson, Dr Donald Lloyd-Jones, Dr Stephen Persell, Dr Sadiya Khan, Dr James Paparello, Ms. Aashima Chopra, Ms. Priya Tripathi, Dr My Vu, Ms. Mianzhao (Tracy) Guo, Ms. Alexandra Soriano, Mx. Rolando Serna, Ms. Patricia Helbin, Mr. Edgar Pizarro, Dr Daneen Woodard, Dr Natasha Pavlovcik, Dr Charity Alikpala, Ms. Katherine McKeough, Ms. Sonya Hopkins, and Ms. Eloisa Lopez. We would like to thank the trial Data and Safety Monitoring Board members include Dr Paul Muntner (chair), Dr Christopher Lindsell, Dr Kenneth Jamerson, Dr Emily Anderson, and Ms. Perla Herrera. We would like to thank members of the QUARTET Australia study team, including Dr Emily Atkins and Dr Anthony Rodgers. We would like to thank George Clinical for providing independent clinical trial monitoring and Sharp Clinical Services for study drug manufacturing. We thank Dr Emmanuel Tetteh from the Center for Public Health Systems Science and the Institute for Clinical and Translational Sciences at Washington University in St Louis for review and edits of the manuscript. The Translational Science Benefits Model and Translating for Impact Toolkit © 2017-2023, created by the Institute of Clinical and Translational Sciences at Washington University in St Louis and available at translationalsciencebenefitsmodel.wustl.edu, is licensed under Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0).
Author contributions
GI, DL, JDC, and MDH conceived, collected, and reviewed data for inclusion in this manuscript. GI drafted the manuscript. DL, ASB, JM, CKC, NRK, OAS, LR, JDC, and MDH provided critical reviews and intellectual contributions to the manuscript. GI takes responsibility for the manuscript and its contents.
Funding statement
The study was supported by the National Heart, Lung, and Blood Institute (R61/R33HL139852), National Center for Advancing Translational Sciences (UL1TR001422 and UL1TR002345), and Northwestern University Feinberg School of Medicine. The funders were not involved in the development of the study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Competing interests
MDH has received travel support from the World Heart Federation and consulting fees from PwC Switzerland. MDH has an appointment at The George Institute for Global Health, which has a patent and license and has received investment funding with the intent to commercialize fixed-dose combination therapy through its social enterprise business, George Medicines. MDH has pending patents for heart failure polypills. CKC has a patent for quarter–dose, quadruple–drug combination therapy for blood pressure lowering. The remaining authors have nothing to disclose.



