Introduction
Clinical research underpins medical advancements, healthcare policies, and patient outcomes. However, recruiting eligible participants remains a significant challenge, often causing delays, additional costs, and reduced data quality due to inadequate sample diversity and size [Reference Gul and Ali1–Reference Carlisle, Kimmelman, Ramsay and MacKinnon3]. Clinical research professionals (CRPs), including coordinators and research assistants, are crucial conduits between investigators and potential participants [Reference Niranjan, Durant and Wenzel4,Reference Speicher, Fromell and Avery5]. They are often the first interaction point for prospective participants, significantly influencing recruitment success, participant diversity, enrollment numbers, and the participants’ understanding and willingness to participate [Reference Huang, Bull, Johnston McKee, Mahon, Harper and Roberts6,Reference Getz, Campo and Kaitin7]. CRPs employ various strategies to address recruitment challenges, including social media outreach, community programs, database optimization, and coordination with clinical teams within healthcare institutions [Reference Frampton, Shepherd, Pickett, Griffiths and Wyatt8–Reference Scott, McComb and Trachtman12]. Each method has advantages and limitations, demanding a nuanced approach to specific research conditions.
A critical aspect of the CRP role is eligibility prescreening, where the CRP reviews medical records, contacts patients and caregivers, and collaborates with the principal investigator to assess eligibility before informed consent [Reference Idnay, Fang, Dreisbach, Marder, Weng and Schnall13]. This phase of the recruitment process aims to ensure potential participants are likely to fulfill the study’s criteria before any informed consent is sought or detailed medical record review is conducted [Reference Penberthy, Dahman, Petkov and DeShazo14]. Its impact extends beyond mere eligibility; it fundamentally affects the scientific integrity, generalizability, and ethical implications associated with the research. Inadequate prescreening can limit access to potential participants or cause unnecessary distress during consent and screening upon discovering ineligibility, which could have been avoided [Reference Idnay, Fang, Dreisbach, Marder, Weng and Schnall13], thereby skewing research outcomes and raising ethical concerns [Reference Sun, Butler and Diallo15].
Faced with these multifaceted challenges, our study addresses the challenges of eligibility prescreening by exploring where CRPs obtain necessary information and what patient information they consider crucial. We aim to streamline prescreening procedures to enhance recruitment for high-quality clinical research. We conducted a comprehensive survey targeting CRPs across various roles and domains to understand their real-world practices and preferences in eligibility prescreening. The insights from this survey will inform the development of advanced prescreening tools and specialized training programs for CRPs. This effort aims to create a more efficient, effective, and ethically grounded clinical research environment, benefiting the medical research community and diverse patient populations.
Methods
Study design and sampling
This study was approved by the Columbia University Institutional Review Board (#AAAD1873). We conducted a prospective online freelisting survey via Qualtrics (Provo, UT) with 150 CRPs from various institutions within and beyond the Columbia University network recruited through institutional listservs and online community forums using stratified, convenience, and snowball sampling to ensure diverse perspectives. Participants were at least 18 years old with CRP experience within the last 12 months and involved in identifying potential participants for recruitment. We used purposive sampling to target three CRP subgroups: neurological disorders research (n = 50), rare diseases research (n = 50), and other disease domains (n = 50). Neurological disorders research was chosen for its diverse conditions (i.e., from more prevalent disorders such as stroke to less common conditions such as frontotemporal dementia) and unique recruitment challenges (e.g., capacity to consent), rare diseases research for its sourcing complexities from limited populations, and other disease domains for broad representation of clinical research contexts.
Freelisting is a qualitative research technique focused on elucidating the spectrum and organizing principles of terms, concepts, or items that a specific community associates with a given subject matter [Reference Weller16,Reference Decker-Palmer, Klodowski and Thompson17]. Participants are asked to list as many items, terms, or concepts related to a designated topic in response to an open-ended question to allow unrestricted listing, providing insights into the collective cognitive structure of the participants [Reference Weller16,Reference Keddem, Barg and Frasso18]. This method captures the richness and diversity of perceptions, offering a multifaceted view of complex subjects. In healthcare research, freelisting identified terminologies related to symptoms, health beliefs, and cultural perspectives on treatment [Reference Auriemma, Lyon, Strelec, Kent, Barg and Halpern19–Reference Fiks, Gafen, Hughes, Hunter and Barg23]. Our study aimed to understand the terms, concepts, and information CRPs consider essential during the eligibility prescreening for participant recruitment.
Data collection
We conducted a 20-minute online freelisting survey (see Supplementary File A) to understand what CRPs consider crucial in evaluating patient eligibility for clinical research. The survey aimed to identify the main sources of information CRPs consult, the patient-specific details they prioritize, and the role of dialogue between CRPs and principal investigators and potential research participants in assessing eligibility. Participants listed information consulted in medical records, inquiries made directly to patients or caregivers, and discussions with principal investigators. A demographic questionnaire was included to identify trends or biases. Participants received a $40 Amazon gift card upon completion.
Data analysis
Descriptive statistics summarized the survey data. We calculated the salience score for each listed item using Anthropac (Lexington, KY) version 4.98, a tool designed to evaluate data from lists. The salience score represents the weighted average of an item’s (inverse) rank across multiple lists, with each list weighted by its number of items [Reference Weller16,Reference Keddem, Barg and Frasso18]. More specifically, the lower an item’s rank on a list (indicating its higher importance), the higher its salience score. Each list contributes to the score in proportion to its total number of items, thereby giving weight to the prevalence of an item in a broader context. The salience score captures an item’s frequency and prominence, providing insights into the perceived importance among respondents.
Responses were stratified into the three primary disease domains: neurological disorders, rare diseases, and other unspecified domains. Information was categorized based on three sources: caregiver or potential participant inquiry, medical records review, and principal investigator inquiry. Four coders, including one primary coder (BI) and three others (JGN, ERG, and ASJ), with domain-specific recruitment experience, classified synonyms and categories. Regular meetings ensured consensus on discrepancies.
This analysis had two sequential levels: first, identifying and categorizing synonyms to consolidate the list and ensure accurate representation of unique concepts; second, grouping categorized synonyms into broader thematic categories. This two-tiered approach provided granular insights and broader patterns, offering a comprehensive understanding of what CRPs consider essential during the eligibility prescreening phase of participant recruitment.
Results
Participant characteristics
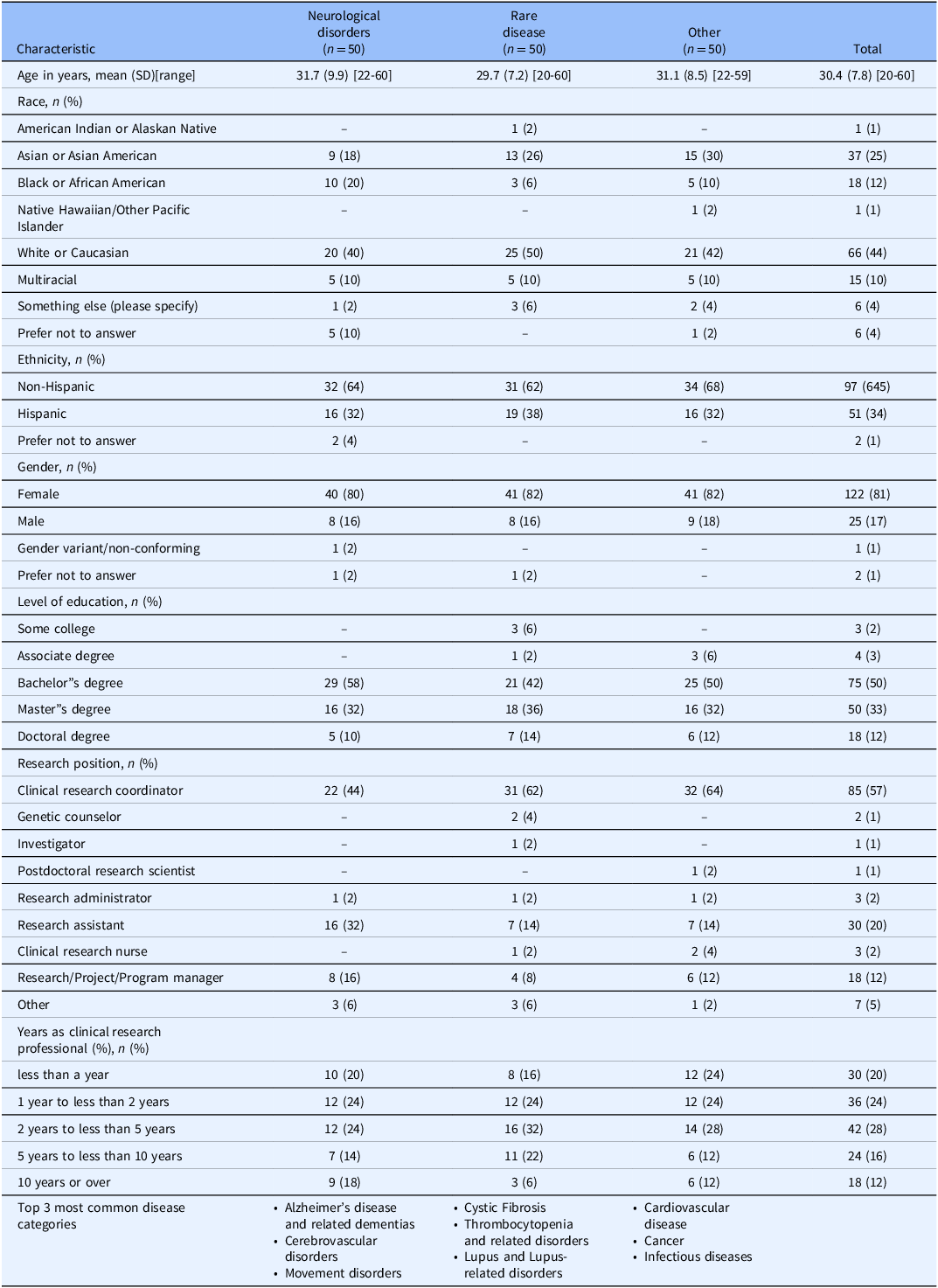
The study included 150 CRPs from diverse disease domains, including neurological disorders, rare diseases, and other areas. Table 1 shows participant demographics: average age was 30.4 years (SD: 7.8; range: 20–60), 81% were female, 44% identified as White or Caucasian, 25% as Asian or Asian American, and 12% as Black or African American. Majority were non-Hispanic (64.5%). Half had a bachelor’s degree, and about one-third had a master’s degree. Predominant roles were clinical research coordinators (57%) and research assistants (20%). Experience in clinical research varied, with about a quarter having one to less than two years or two to less than five years of experience.
Table 1. Participant characteristics (N = 150)
Synonyms and Categories Identified During Potential Participant Inquiry.
Supplementary Table S1 details categories and synonyms. In neurological disorders, 15 categories were identified, with “History of present illness” being the most prevalent, including cognitive status to disease onset age. “Research participation” highlighted aspects of readiness for research. The rare diseases domain revealed 14 categories, some overlapping with neurological disorders, with unique specifics like the last menstrual period in “Obstetric and gynecological history” and Raynaud’s syndrome in “Past medical history.” Other disease domains had 17 categories. “History of present illness” covered aspects like symptom duration, while unique categories like “Physical activity status” examined activity level. “Social History” emphasized factors like incarceration, highlighting various social elements affecting health or research eligibility.
Synonyms and categories identified during medical records review
In neurological disorders, 16 categories were identified (Supplementary Table S2). “Clinical care history” included healthcare provider and hospitalization, while “Cognitive test results” comprised Mini-Mental Status Examination. “Personal information” included information such as name, and “Research Eligibility” and “Research participation” spotlighted research adherence, and family willingness, respectively. The rare disease domain listed 18 categories, overlapping with neurological disorders in “Clinical care history” and “History of present illness,” but with unique terms including “lung infection” and “Mortality.” Other disease domains had 17 categories, with “Diagnostic results” covering genetic results, and “Obstetric and gynecological history” including conception method. “Research eligibility” covered adherence to treatment regimens.
Synonyms and categories identified during principal investigator inquiry
In neurological diseases, 17 categories were identified (Supplementary Table S3), covering clinical care to research aspects. “Clinical care history” included terms like healthcare provider, while “Research participation” covered study logistics and patient willingness. Rare diseases had 14 categories, with unique terms like fetal abnormalities in “Obstetric and gynecological history.” “Harmful substance use” covered alcohol and smoking histories, highlighting lifestyle impacts on disease. Other disease domains showcased 16 categories, with diverse “History of present illness,” focusing on diagnosis to symptom duration, “Physical activity status” on mobility, and a new “Recruitment” category reflected patient engagement procedures.
Similarities and differences in information gathering by disease domain
For neurological diseases, respondents used similar information sources (i.e., potential research participant, medical records, and principal investigator) to inquire about clinical care history, clinical documentation, demographics, diagnostic results, family history, history of present illness, past medical history, personal information, research eligibility, research participation, treatment or medication, and visit data. Cognitive test results were reviewed through medical records review and principal investigator inquiries, delving into mental status, mood, and prognosis.
For rare diseases, demographics, diagnostic results, history of present illness, obstetric and gynecological history, past medical history, personal information, research eligibility, research participation, treatment or medication, and visit data are consistent across all sources. Clinical care history was only inquired about from the medical records and potential participant inquiries. Physical function and harmful substance use were comprehensively examined across all data sources.
Regarding other diseases, common categories across all stages were clinical care history, demographics, diagnostic results, history of present illness, obstetric and gynecological history, past medical history, personal information, research eligibility, research participation, harmful substance use, treatment or medication, and visit data. The principal investigator inquiry included nuances like surgical candidacy and the need for dental screenings or glucose management, which were less prominent in other domains.
Core categories were consistent across stages and domains, but the depth and specificity of inquiries varied. The participant inquiry focused on broader categories, the medical records review provided detailed clinical history, and the principal investigator inquiry delved deeper into aspects critical for research participation and the disease.
First-level analysis: synonyms by disease domain
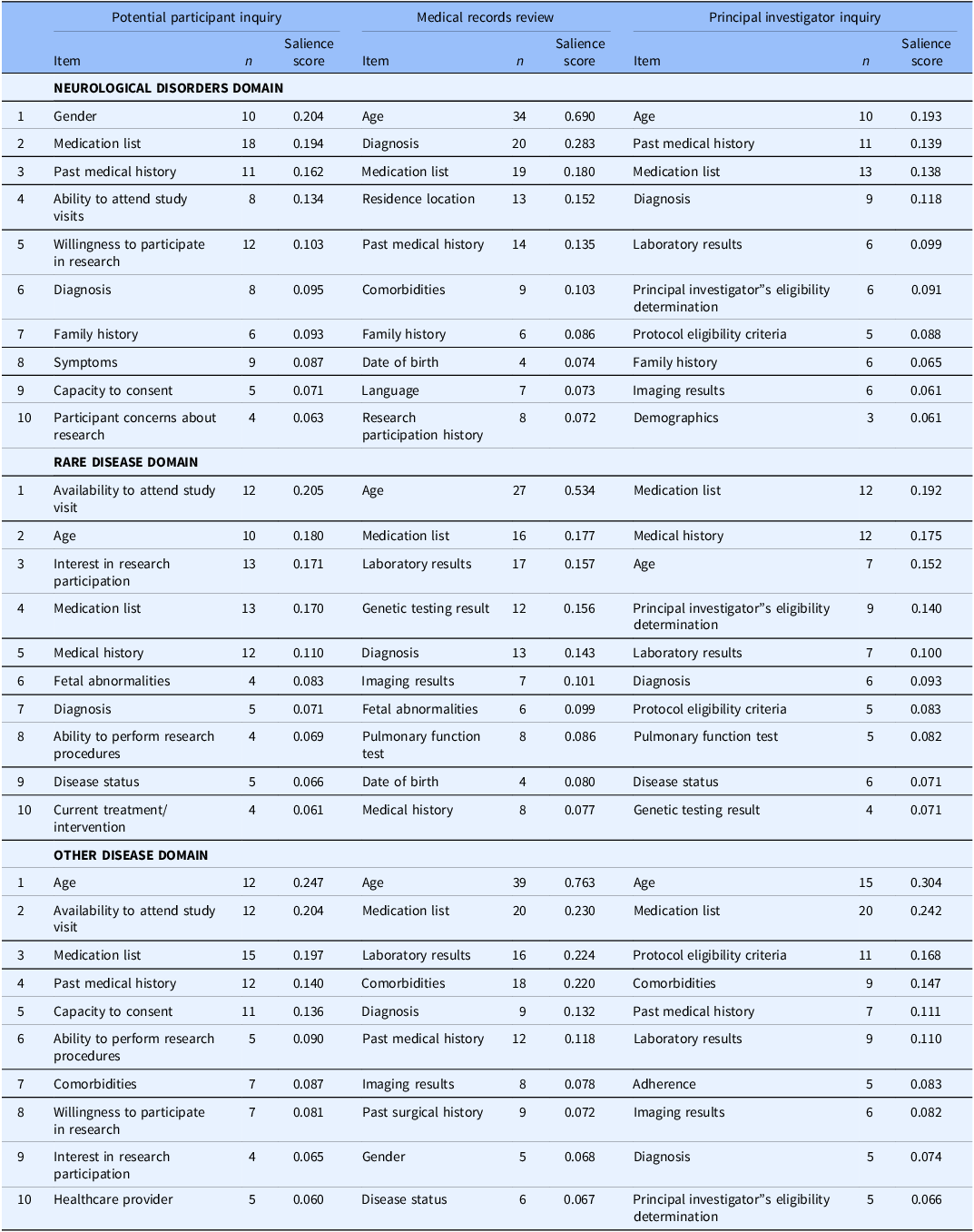
The first level of analysis categorized items by synonyms, revealing distinct patterns of information prioritization among CRPs across three disease domains (Table 2). For detailed salience scores and items, see Supplementary Tables S4–S6. “Age” was the most salient item in neurological disease during medical records reviews (0.690). “Gender” and “Age” topped potential participant inquiries and principal investigator discussions, scoring 0.204 and 0.193, respectively. “Medication list” and “Medical history” were also emphasized across all categories.
Table 2. Top 10 synonyms by disease domain: a first-level analysis of salience scores
Second-Level Analysis: Categorization by Diseases Domain.
In rare diseases, “Availability to attend study visit” was most salient in potential participant inquiries (0.205), followed by “Age” (0.180) and “Interest in research participation” (0.171). “Age” was prioritized in medical records reviews (0.534), followed by “Medication list” (0.177) and “Laboratory results” (0.157). Principal investigator inquiries focused on the “Medication list” (0.192), “Medical history” (0.175), and “Age” (0.152).
For other diseases, potential participant inquiries highlighted “Age” (0.247), “Availability to attend study visit” (0.204), and “Medication list” (0.197). Medical records reviews were dominated by “Age” (0.763), succeeded by “Medication list” (0.230) and “Laboratory results” (0.224). Principal investigator inquiries emphasized “Age” (0.304), “Medication list” (0.242), and “Protocol eligibility criteria” (0.168).
A key similarity across all domains (Figure 1) was the high importance of “Age” and the commonality of “Medication List.” “Past Medical History” and “Diagnosis” also consistently appearing, albeit at varying ranks. Differences were evident in the emphasis on specific factors within each disease domain. In the neurological disorders, potential participant inquiries highlighted “Ability to attend study visits” and “Willingness to participate in research,” with medical records review often including “Residence location” and “Comorbidities.”

Figure 1. Shared and divergent information inquiry from data sources across domains.
In rare diseases, potential participant inquiries and medical records reviews uniquely highlighted “Fetal abnormalities.” The medical records review and the principal investigator’s inquiry mentioned “Genetic testing result” and “Pulmonary function test.” This domain did not saliently inquire about “Past medical history” from potential participants, nor did “Family history” rank in the top ten most salient terms, but “Genetic testing results” were emphasized.
For other diseases, potential participant inquiry prioritized “Availability to attend study visit,” while medical records review focused on “Comorbidities” and “Past surgical history.” Principal investigator inquiries in this domain distinctively featured “Adherence.”
Overall, these variations revealed each inquiry method’s differing perspectives and priorities. Potential participant inquiries focused on participation feasibility and willingness factors, medical record reviews emphasized historical and diagnostic data, and principal investigator inquiries balanced logistical aspects like “Medication list” and specific medical details like “Laboratory results” and “Diagnosis.”
In our second-level analysis, detailed in Table 3, we explored the frequency and salience of categories within three disease domains. In neurological disorders, “History of present illness” was significant in potential participant and principal investigator inquiries, with salience scores of 0.394 and 0.334, respectively. Interestingly, “Diagnostic results” were exclusively reviewed from the medical records and inquired from the principal investigator but were not asked from potential participants. “Harmful substance use” was examined only from potential participants. Demographic information was primarily sought during medical records reviews, with a salience score of 0.706.
Table 3. Categories by disease domain: a second-level analysis of salience scores

Note: OBGYN: Obstetrics and gynecology.
In rare diseases, “Research participation” was most salient in potential participant inquiries (0.447). Demographic information, “Mortality,” and “Recruitment” were primarily sought during medical records reviews. “Family history” and “Clinical documentation” were not discussed with principal investigators.
In other disease domains, “Research participation” and “Demographics” were highly frequent and salient across all aspects of clinical research. “Contact information” was asked only from potential participants, and “Recruitment” was exclusively requested from the principal investigator. “Family history” was notably absent in principal investigator inquiries.
“Demographics” and “History of present illness” were consistently significant across domains. Unique patterns were observed, such as “Diagnostic Results” and “Harmful substance use” in neurological disorders, which are context-specific, providing a nuanced understanding of category relevance in clinical research.
Discussion
This study used a freelisting survey to investigate CRPs perspectives across neurological disorders, rare diseases, and other disease domains, engaging 150 CRPs to delineate the complexities of eligibility prescreening. This approach enriched the investigation by identifying and prioritizing elements deemed most important by CRPs [Reference Weller16,Reference Decker-Palmer, Klodowski and Thompson17]. The results highlighted the diversity and complexity of prescreening procedures across disease domains, emphasizing the need for tailored approaches in clinical research.
First-level analysis revealed that age consistently emerged as crucial information across all domains and inquiry methods, influencing participant selection, treatment approach, and study outcomes. Medication lists were also salient, indicating the significance of understanding participants’ concurrent therapies. Each disease domain showcased unique priorities, with neurological disorders focusing on logistical considerations, participants’ research inclination, and rare diseases prioritizing genetics. This juxtaposition highlights the necessity of domain-specific knowledge and the tailored approach required in clinical research. Inquiry methods varied, with potential participant inquiries focusing on participation feasibility, medical record reviews on historical data, and principal investigator inquiries combining both. This divergence illuminates the multifaceted nature of clinical research and underscores the importance of a holistic approach, integrating both logistical and medical considerations, for optimal research outcomes.
The second-level analysis demonstrates that “History of present illness” was prioritized in neurological disorders, especially in participant and principal investigator inquiries. At the same time, “Diagnostic results” were focal during medical records reviews and principal investigator inquiries. “Harmful substance use” was exclusively inquired during participant interactions. The difference between the specific categories queried and the consistent focus on demographic data in medical records reviews highlights how CRPs prioritize the information they seek depending on the source, using this strategy to make eligibility prescreening more efficient. In rare diseases, “Research participation” was crucial in participant inquiries, while “Mortality” was exclusively explored in medical records reviews. The limited focus on family history and clinical documentation in principal investigator inquiries highlighted the genetic emphasis in rare disease trials. In other disease domains, “Research participation” and “Demographics” were generally salient, with specific inquiries into contact information and recruitment reflecting varied informational demands. Additionally, it is noteworthy that there is a conspicuous absence of attention to race and ethnicity, especially given that many trials now set caps based on these factors [Reference Turner, Steinberg, Weeks, Rodriguez and Cullen24]. Considering socioeconomic status, education, and an individual’s capacity to participate is equally crucial, reflecting the evolving landscape of trial prerequisites [Reference Idnay, Fang and Butler25]. The specific patient-oriented inquiries into contact information and principal investigator-exclusive discussions around recruitment offer a peek into the varied, stakeholder-specific informational demands and necessitate a reflective consideration of tailored communication strategies.
These findings underscore the multifaceted nature of clinical research and the need for a holistic approach integrating logistical and medical considerations. Based on these insights, we propose thematic recommendations (Table 4) to develop training and frameworks, ensuring robust and specialized recruitment strategies tailored to each domain. These findings also pave the way for a fine-tuned, domain-oriented approach to developing training and frameworks, ensuring that recruitment strategies are universally robust and aptly specialized [Reference Palac, Alam, Kaiser, Ciolino, Lattie and Mohr26].
Table 4. Recommended practices for clinical research eligibility prescreening

Note: CRP: clinical research professional.
Implications for clinical research practices
Given the universal and domain-specific elements in eligibility prescreening, our findings highlight the need for a refined approach to clinical research practices, particularly regarding participant prescreening strategies. The consistent importance of “Age,” “Medication list,” and “Medical history” across research domains posits these as essential starting points. Nevertheless, distinct nuances within each domain require a bifurcated strategy, blending universal principles with adaptability to domain-specific variables.
Extending from Miller et al.’s study [Reference Miller, Gleason and Juraschek27] on demographics in research participant selection, our findings suggest that while demographic data, such as age and gender, are vital, their salience varies across domains. Acknowledging the overarching and domain-specific components, it becomes imperative to integrate a multifaceted strategy into clinical research practices by reconfiguring existing frameworks and training modules to embody a more nuanced, contextually relevant approach that resonates with the shared cognitive schemas among CRPs and is congruent with the specificities intrinsic to each research domain. The “one-size-fits-all” approach to CRP training risks impeding the nuanced operation required in distinct research domains [Reference Speicher, Fromell and Avery5,Reference Idnay, Fang, Dreisbach, Marder, Weng and Schnall13]. Specialized training and resources will help CRPs understand and implement domain-specific variations.
Enhancing resources and tools to encapsulate these findings is paramount. Improving eligibility prescreening tools to incorporate generalized prescreening criteria with an adjustable interface for domain-specific variables could offer CRPs a flexible platform [Reference Fang, Idnay and Sun28]. These tools, integrated with informatics capabilities, can evolve in real time, aligning prescreening criteria with emerging trends [Reference Idnay, Dreisbach, Weng and Schnall29]. Continuous professional development, including workshops and webinars on prescreening practices, will strengthen CRPs’ ability to navigate participant recruitment adeptly and effectively. Embedding these enriched training paradigms and dynamic resources will improve the precision and efficiency of prescreening strategies, fostering continuous learning and adaptability in CRPs’ practice.
Ethical considerations and participant-centered approach
Our research emphasizes a participant-focused approach in clinical studies, underscoring the importance of respecting each participant’s unique health context. Ethical considerations are central to the prescreening process, merging scientific rigor with a dedication to participant well-being [Reference Salloch, Schildmann and Vollmann30]. Beyond determining eligibility, ethical considerations ensure scientific integrity and participant safety. Detailed understanding of participants’ medical histories is essential, both operationally and ethically, to prevent unintentional harm.
This ethical duty involves assessing risks and benefits for participants through a detailed insight into their medical histories to mitigate potential risks preemptively. A participant-centered model incorporates patient perspectives and experiences, meets ethical standards, and reflects participants’ realities. For example, understanding the challenges of attending study visits, as shown in our findings, urges researchers to create feasible and compassionate participant pathways. Consent forms should be crafted at a 5th-grade reading level to ensure accessibility and comprehension [Reference González-Duarte, Zambrano-González and Medina-Franco31]. Data privacy and autonomy are crucial, given the personal information collected during prescreening. Stringent data protection measures and clear communication regarding data handling are vital to maintaining ethical standards [Reference Bender, Cyr, Arbuckle and Ferris32].
Limitations and future directions
While our study provides invaluable insights, it has limitations. The sample, although diverse, may not capture the full spectrum of CRP roles and experiences. Additionally, the systematic and thorough categorization and analysis are subject to coders’ interpretation. Future research should expand the participant pool and explore additional research domains, diverse geographic and institutional contexts, and varying CRP roles. Investigating how different institutional practices, guidelines, and cultures influence prescreening strategies could unravel more nuanced insights.
Additionally, while freelisting provides a structured mechanism to extract salient factors from participants’ responses, it may inadvertently omit subtle yet critical nuances and contexts that permeate CRPs’ experiences and decision-making. Future research should focus on the social determinants of health to ensure a more inclusive approach. Further, based on our findings, we plan to evaluate the effectiveness of CRPs’ prescreening practices.
Moreover, while the online survey offers a broad reach, it may have predisposed the findings to certain biases. Technological familiarity, digital literacy, and the absence of real-time clarification or probing might have shaped the responses, potentially skewing the results toward a more digitally comfortable and articulate demographic.
Finally, the intersection of technology and clinical research prescreening presents a fertile ground for exploration and innovation. Investigating the impacts and utilities of digital health tools, electronic health records (EHRs), artificial intelligence (AI), and predictive analytics in optimizing the prescreening process could pave the way for developing automated, efficient, and sophisticated eligibility determination strategies. For instance, integrating AI and predictive analytics with EHRs could automate the initial stages of eligibility screening, identifying potential participants based on predefined criteria and enabling CRPs to focus on more nuanced and context-specific prescreening considerations.
Conclusion
This study offers a comprehensive and nuanced perspective on the practices and preferences of CRPs in the pivotal task of eligibility prescreening across diverse disease domains. Identifying universal and domain-specific elements lays the foundation for refining recruitment strategies, enhancing training programs, and developing tailored resources. The balance between generalized approaches and specialized nuances is crucial for fostering a clinical research environment that is ethical, efficient, and participant-centered. Through these insights, we contribute to the ongoing endeavor to advance clinical research practices to improve patient outcomes and medical advancements.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cts.2024.617.
Author contributions
BI: conceptualization, methodology, formal analysis, investigation, data curation, writing - original draft, visualization, and project administration; ERG: validation, formal analysis, and writing - review & editing; ASJ: validation, formal analysis, and writing - review & editing; JGN: validation, formal analysis, and writing - review & editing; KM: validation, resources, and writing - review & editing; CW: conceptualization, resources, writing - review & editing, supervision, and funding acquisition.
Funding statement
This work was supported by the National Library of Medicine grants R01LM009886 and T15LM007079 and the National Center for Advancing Clinical and Translational Science grant UL1TR001873. The content is solely the authors’ responsibility and does not represent the official views of the National Institutes of Health.
Competing interests
No conflicts of interest to disclose.







