No CrossRef data available.
Published online by Cambridge University Press: 26 March 2019
OBJECTIVES/SPECIFIC AIMS: The first aim of the study is to evaluate the accuracy of serum biomarkers of acute GVHD measured after four weeks of corticosteroid therapy to predict 6 month NRM. The second aim of this study is to compare the accuracy of the biomarker algorithm to that of clinical response to corticosteroids after four weeks. The third aim of the study is to develop a novel regression model that uses weekly biomarker measurements over the first month of corticosteroid therapy to predict 6 month NRM. METHODS/STUDY POPULATION:. Patients who received HCT at one of 22 IRB-approved centers and provided blood samples to the Mount Sinai Acute GVHD International Consortium (MAGIC) biorepository and developed GVHD between January 2008 to May 2018 are included in this study. Patients were divided by time into a training set (Jan 2008-Dec 2015, n=233) for model development and a validation set (Jan 2015-May 2018, n=357) to evaluate the predictive performance of the model. The later time of the validation set was chosen deliberately to model contemporaneous GVHD treatment practices. The size of each group was designed so that there would be roughly equal numbers of deaths in both groups. RESULTS/ANTICIPATED RESULTS:. Serum concentrations of GVHD biomarkers after one month of corticosteroid therapy were measured in the validation set, and the predicted probability of NRM (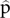 $\hat{\rm p}$) was computed according to the previously published algorithm:
$\hat{\rm p}$) was computed according to the previously published algorithm: 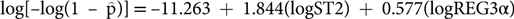 $\log[-\log(1 - \hat{\rm p})]=-11.263 + 1.844({\rm logST}2)+ 0.577({\rm logREG}3\alpha)$. The performance of the biomarker algorithm was evaluated by creating receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) in the validation set. The AUC of the biomarker algorithm was a significantly better predictor of 6 month NRM than clinical response to treatment after four weeks of corticosteroids (0.84 vs. 0.64, p<0.001), which is a clinically relevant improvement in accuracy. To evaluate serial biomarker monitoring, serum biomarker concentrations will be measured weekly at five time points from treatment initiation to one month after corticosteroid therapy. We will use these values in the training set to develop a regression model for 6 month NRM that accounts for repeated biomarker measurements. The performance of this model will be tested in the validation set and the accuracy of the serial biomarker measurements will be compared to the accuracy of measuring biomarkers at the single time point after four weeks of corticosteroid therapy. An AUC improvement of 0.05 would be considered clinically significant. DISCUSSION/SIGNIFICANCE OF IMPACT: Clinical response to treatment after four weeks has been the standard endpoint in GVHD interventional trials for decades. If biomarkers measured at the same time more accurately predict long term mortality, this study would provide the basis for a novel endpoint in GVHD trials and enable more accurate determination of effect size of experimental interventions. An accurate biomarker algorithm will prove useful in guiding immunosuppressive treatment decisions for patients with GVHD. Patients identified by the algorithm as low-risk may benefit from reduced-dose corticosteroid therapy, potentially reducing lethal opportunistic infections. Patients identified as high-risk will be candidates for more intensive immunosuppression or investigational therapies. This precision medicine approach tailors therapy to the individual patient’s biology.
$\log[-\log(1 - \hat{\rm p})]=-11.263 + 1.844({\rm logST}2)+ 0.577({\rm logREG}3\alpha)$. The performance of the biomarker algorithm was evaluated by creating receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) in the validation set. The AUC of the biomarker algorithm was a significantly better predictor of 6 month NRM than clinical response to treatment after four weeks of corticosteroids (0.84 vs. 0.64, p<0.001), which is a clinically relevant improvement in accuracy. To evaluate serial biomarker monitoring, serum biomarker concentrations will be measured weekly at five time points from treatment initiation to one month after corticosteroid therapy. We will use these values in the training set to develop a regression model for 6 month NRM that accounts for repeated biomarker measurements. The performance of this model will be tested in the validation set and the accuracy of the serial biomarker measurements will be compared to the accuracy of measuring biomarkers at the single time point after four weeks of corticosteroid therapy. An AUC improvement of 0.05 would be considered clinically significant. DISCUSSION/SIGNIFICANCE OF IMPACT: Clinical response to treatment after four weeks has been the standard endpoint in GVHD interventional trials for decades. If biomarkers measured at the same time more accurately predict long term mortality, this study would provide the basis for a novel endpoint in GVHD trials and enable more accurate determination of effect size of experimental interventions. An accurate biomarker algorithm will prove useful in guiding immunosuppressive treatment decisions for patients with GVHD. Patients identified by the algorithm as low-risk may benefit from reduced-dose corticosteroid therapy, potentially reducing lethal opportunistic infections. Patients identified as high-risk will be candidates for more intensive immunosuppression or investigational therapies. This precision medicine approach tailors therapy to the individual patient’s biology.