Introduction
The ‘livestock revolution’ has seen the lives and livelihoods of peri-urban peoples increasingly intertwine with pigs and poultry across Africa (Figué and Desvaux Reference Figué, Desvaux, Morand, Lefait-Robin and Apiwathnasorn2015). A phrase coined to describe the expansion of animal protein production in order to meet rising demand for meat from populations with increasing purchasing power (Delgado et al. Reference Delgado, Rosegrant, Steinfeld, Ehui and Courbois2001), the idea of the livestock revolution promised a development solution – lifting people out of chronic poverty through production of meat – as well as a health solution through improved nutrition via protein consumption (Sumberg and Thompson Reference Sumberg and Thompson2013). At the same time the scale and speed of farming intensification has sparked concern, including the potential for antibiotic misuse and its consequences for antibiotic resistance in humans, a problem of significant scale globally (Murray et al. Reference Murray, K. Shunji Ikuta, L. Swetschinski, A. Gray, C. Bisignano, E. Wool, Johnson, Browne, F. Fell, G. Haines-Woodhouse, Kashef Hamadani, Kumaran, R. Agarwal, S. Albertson, J. Andrews, E. Ashley, S. Baker, A. Bekker, A. Bethou, S. Boonkasidecha, C. Carvalheiro, V. Chansamouth, S. Chiurchiù, Cook, B. Cooper, Cressey, M. Cunningham, Day, M, De Luca, A. Dramowski, Dunachie, D. Eibach, N. Feasey, K. Forrest, P. Gastmeier, R.C. Greer, S. Haller, Hay, M. Holm, Iregbu, J. Jacobs, F. Javanmardi, N. Kissoon, T. Kostyanev, R. Krumkamp, H. Hmwe Kyu, D. Limmathurotsakul, M. Lunn, N. Mturi, P. Musicha, T. Nakamura, S. Nangia, C. Ngoun, D. Nwakanma, Obiero, P. Olliaro, E. Ortiz-Brizuela, C. Perrone, A. Ponce-de-Leon, T. Ramdin, T. Roberts, A. Roca, Rudd, J. Schnall, M. Shivamallappa, N. Steenkeste, T. Stoeva, A. Thaiprakong, Turner, P. Turner, S. Velaphi, H. Vu, S. Waner, T. Wozniak, B. Sartorius, Lopez, Moore, Dolecek and Naghavi2022). Farmers are now implored to cut back on antibiotic use, in particular the use of antibiotics of medical importance in human health (World Health Organisation 2017).
In food production, antibiotic use became commonplace in intensified livestock farming practices around the globe through the second part of the twentieth century (Kirchhelle Reference Kirchhelle2018; Podolsky Reference Podolsky2015; Silbergeld Reference Silbergeld2019; Hinchliffe, Butcher, and Muhammad Reference Hinchliffe, Butcher and Muhammad2018). Projections suggest that global antimicrobial use in food animals will rise by 67%, to 105,596 (±3,605) tons by 2030 (Van Boeckel et al. Reference Van Boeckel, C. Brower, Grenfell, Levin, Robinson, Teillant and Laxminarayan2015). Sub-Saharan Africa, including Uganda, has seen a steady increase in antibiotic use over the past three decades (Silbergeld Reference Silbergeld2019). This trend has raised alarm amongst those concerned with antimicrobial resistance (AMR) and its consequences (Silbergeld Reference Silbergeld2019). Many of the antibiotics used in animal production are critically important for human health, for example macrolides and polymixins (Manyi-Loh et al. Reference Manyi-Loh, Mamphweli, Meyer and Okoh2018; Collignon et al. Reference Collignon, Powers, Chiller, Aidara-Kane and Aarestrup2009). Characterised as a critical threat to human health, economies and security globally (O’Neill Reference O’Neill2016; World Health Organisation 2015), rising antibiotic resistance has become a core concern at the intersection of the production of animal meat and human livelihoods (Food and Agricultural Organization 2019).
AMR is being tackled under a One Health umbrella, recognising the importance of interconnections between humans, animals and environments (Chandler Reference Chandler2019) and has been characterised as an intrinsically ‘biosocial’ problem (Kirchhelle et al. Reference Kirchhelle, P. Atkinson, K. Chuengsatiansup, N. Fortané, C. Gradmann, Hoffman, J. Lezaun, K. Outterson, Podolsky, Roberts, Singer, So, L. Sringernyuang, Rogers Van Katwyk and Chandler2020). Scholars have drawn attention to the need to understand the changes driving AMR as properties of anthropogenic ways of life in the modern era (Lee and Motzkau Reference Lee and Motzkau2013) that reflect a micro-biopolitical conundrum where the agendas of microbes, farmers, publics, authorities and transnational agencies are in tension (Hinchliffe, Butcher, and Muhammad Reference Hinchliffe, Butcher and Muhammad2018). To understand the ways these different logics play out requires close attention to the practices, principles and potentials held between people, animals, microbes, markets and policies (Brives, Rest, and Sariola Reference Brives, Rest and Sariola2021). Taking a biosocial perspective enables analysis that transects the health and wellbeing of humans and the health and wellbeing of animals, and illustrates the ways in which substances imagined primarily as human health commodities can take on wider economic and political significance. This paper focuses on the realities for farmers in peri-Urban Wakiso, Uganda, of taking the opportunities presented by the livestock revolution, and the reasons that antibiotics will become difficult to disentangle from this venture.
In Uganda, agriculture is a critical economic activity, contributing about 24.6 percent to GDP and 71 percent to employment (Food and Agricultural Organization 2019). For decades, farmers have been encouraged to adopt ‘improved’ livestock and poultry breeds so as to enable a shift from subsistence to market based agriculture (Government of Uganda 2005). Recently, the expansion of livestock farming has occurred not only in rural areas but in peri-urban settings surrounding cities, primed for investment in mid-scale commercial farming to serve the increasing urban population (Food and Agriculture Organization 2018). Here, livestock offer a ‘bank account’ for families needing to buffer uncertain conditions of life and livelihoods (Thompson Reference Thompson2021). Intensification of livestock production in these peri-urban settings has raised concern for zoonotic disease as well as antimicrobial resistance (Latino, Pica-Ciamarra, and Wisser Reference Latino, Pica-Ciamarra and Wisser2020). In Wakiso, a peri-urban district surrounding Kampala, a rapid and substantial increase in pig and poultry farming has occurred, to meet apparent demand of city dwellers. This expansion occurs alongside an increasing move towards entrepreneurial solutions to poverty (Pfeilstetter Reference Pfeilstetter2022) – whereby innate entrepreneurial energy is not only to be unleashed but farmers are also to be cultivated into micro-entrepreneurs through training, disciplining and transformation (Dolan and Rajak Reference Dolan and Rajak2016). This research traced the significance of the entrepreneurial mode of what we term quick farming – a phenomenon that combines a promise of easy income through rapid production of ‘exotic’ pigs and poultry with imported methods and measures for production in small physical spaces. An analysis of what it takes to succeed in quick farming, and the impact of these approaches on lives and livelihoods, is presented. The tension between the entrepreneurial promise of quick farming and the lived realities of the risks and responsibilities involved in making this work produces a requirement for forms of investment protection, including the use of antibiotics. As such, this paper argues that these medicines have become a tool to manage the disjuncture between the evasive promise of a better life and the actual experience of quick farming animals in precarious and non-adapted settings. As such, antibiotics have become embedded in the livestock revolution in the entrepreneurial era.
Methods
Ethnographic research was carried out between May 2018 and March 2021. First, a ‘drug bag’ survey was conducted with farmers at 115 farms – 51 pig, 50 poultry and 14 mixed – in six urban town councils of Wakiso district including Kira, Kasangati, Kyengera, Katabi, Katabi and Makindye Ssabagabo (Figure 1). Respondents were presented with samples of antibiotics acquired from within the study area to ascertain recognition of different medicines, frequency of use and source of medicines. As described elsewhere (Dixon et al. Reference Dixon, MacPherson, Manyau, Nayiga, Khine Zaw, Kayendeke, Nabirye, Denyer Willis, de Lima Hutchison and Chandler2019), the method provided an entry point for more in-depth research. Second, 18 weeks of participant observation was carried out at three pig and three poultry farms selected from those involved in the survey for their willingness to participate and to cover a variety of their levels of intensification and duration of engagement in farming. MK observed and participated in daily routines including preparation of feeds, feeding animals, screening the health of animals, treating animals, vaccinating animals and purchasing farm supplies, picking eggs and cleaning animal houses. She typed and shared detailed field notes, and throughout, the whole study team were examining socio-material relations and economic decisions both on the farms and with the wider network of farm supply and market chains (Bernard Reference Bernard2011; Whyte, Van der Geest, and Hardon Reference Whyte, Van der Geest and Hardon2002), which included attending one public event promoting farming practices and materials. Third, 34 key informant interviews were carried out to explore further the themes arising in the ongoing intermittent on-farm participant observation and the earlier survey, as well as to gather oral histories of the development of the agriculture sector. Interviewees included 17 pig and poultry farmers, 6 local animals feed mixers and sellers, 9 stakeholders in the agricultural sector and 2 oral histories with retired agricultural sector stakeholders. A series of feedback focus group discussions (FGDs) were held to present and discuss interim findings from the study and seek further clarification for ongoing analysis: three with 38 farmers in three areas in Wakiso district and one with 7 veterinary officers from across the district. Fourth, documentary analysis was carried out on both present day and historical materials. This included gathering publicly available media articles and policy reports relevant to farming and antibiotic use in the study area from May 2018, and copying relevant archival materials from the Ugandan National Archives.

Figure 1. (a) Location of Wakiso in Uganda, and (b) Participating farms in Wakiso.
Data analysis was ongoing through the study, in collaboration with the study team as well as research participants. Qualitative data were transcribed and translated where required, and uploaded into Nvivo 12 (QSR International). Media and archival materials were also uploaded into the NVivo software for analysis. The materials were coded line by line and organised into themes that emerged from across the wide set of data, in conversation with anthropological scholarship on the topics of antibiotics, development and farming in Africa.
Results
The findings begin with a brief history of livestock programmes in Uganda, followed by a description of what this article terms the quick farming phenomenon. This explains the significance of quick farming as part of a peri-urban promissory assemblage, creating an appetite and opportunities to invest in capital, land, infrastructure, labour and human resources. The article juxtaposes this promise with the numerous risks and responsibilities that are shouldered by those taking on these projects. It highlights how antibiotics emerge as a form of protection in the quick farming enterprise.
Livestock programmes, exotic breeds and antibiotics for livestock in Uganda
During British colonial rule, the potential for commercialised livestock production was a concern that drove the development of Western veterinary medicine in Africa (Anderson Reference Anderson, Brown and Gilfoyle2010). Farmers were encouraged to adopt ‘progressive’ farming practices, although authorities were often disappointed by the inability or unwillingness of local farmers to adopt these. A 1929 exchange of letters between the Director of Veterinary Services in Uganda and the Chief Secretary (Perryman Reference Perryman1929) shows a debate over whether the Soroti stock farm, charged with demonstrating how to farm commercially and supplying breeding material for ‘progressive’ farms, should remain under ‘native’ administration or if the protectorate should take over. The letters conclude that, as native management was deemed less effective, it would be more profitable for the government to take control. The importance of progression continues in a ‘Review of Nutrition’ that was carried out by the Medical Department of Uganda in 1949 (Uganda Medical Department 1945) in which the production of livestock was declared ‘problematic’ due to the over-consumption of supplies, tsetse flies and trypanosomiasis and ‘the relative neglect of animal husbandry.’ To overcome this, the review suggested a series of measures by the Veterinary Department including the ‘steady development of the market system’ and the active encouragement of pig breeding. The concept of ‘progressive’ farming - a narrative of colonial agents - was applied also to progressive farmers, who not only would use ‘improved’ techniques and stock, but were characterised in 1969 as ‘willing to experiment and try out new ideas; he visits Kampala more frequently than the other farmers… to have some kind of work training… has wider contacts with local administrative, government and farming officials; is more likely to visit farm institutes, research station and has more contact with the outside world through radio and newspapers’ (p56) (Bowden and Moris Reference Bowden and Moris1969). Thus, notions of farming promoted by colonial agents were entangled with wider notions of social progression and projects of modernity in Uganda as elsewhere in the region (Leo Reference Leo1978; Mapila, Makwenda, and Chitete Reference Mapila, Makwenda and Chitete2010). The newly independent Ugandan government was, from the early 1960s, widely promoting commercialised farming, and supporting this with wide cadre of government-funded veterinary staff including veterinary assistants and animal husbandry officers, as well as veterinary scouts, field assistants and field officers (Silkin and Kasirye Reference Silkin and Kasirye2002). This situation of veterinary services and animal health policies is common within post colonial and post communist countries (Jaime, Hobeika, and Figuié 2020; Ruhlmann Reference Ruhlmann2018; Figuié, Binot, and Caron Reference Figuié, Binot and Caron2015). However, this ‘good quality veterinary service,’ as described by one of the interviewees who had served as a veterinarian since the end of colonial times, was not to last. The political and economic deterioration from 1971 to 1985 left a skeletal veterinary service, intermittent supplies of veterinary drugs and the creation of a black market. With the liberalisation of the veterinary drug supply in 1976 by the Ministry of Agriculture, farmers were able to access classified drugs and treat their animals themselves (Silkin and Kasirye Reference Silkin and Kasirye2002). Privatisation of government clinical veterinary services occurred from the late 1980s in line with loan conditions for structural adjustment, and successive restructuring reduced ministry staff from 1431 posts to 281 by 1998, with 80 private veterinary practices established over the same period (Silkin and Kasirye Reference Silkin and Kasirye2002). In the absence of a formalised veterinary system, non-governmental organisations (NGOs) began to fill the gap, each with different foci and techniques. A series of programmes to improve livestock production ensued, including a World Bank loan for a Livestock Services Project; an EU-funded privatisation programme; then as part of the Poverty Eradication Action Plan, a pillar on the Plan for Modernization of Agriculture, aiming to transform subsistence farming into commercial farming including through privatisation, marketisation and the adoption of new technologies (Ministry of Finance 2000). In 2001, the government established the National Agricultural Advisory Service (NAADS) programme as a public-private initiative, with the goal of increasing market-oriented agricultural production by addressing through advisory services constraints to agricultural development, seen as a lack of access to information, knowledge and improved technology. Where it worked, the programme seemed to increase the uptake of farming enterprises, including diversification to different products, but overall yield was not increased (Benin et al. Reference Benin, Nkonya, Okecho, Pender, Nahdy, Mugarura, Kato and Kayobyo2007). Most recently, in 2013 agricultural reform was organised under Operation Wealth Creation, with the main goal of reducing poverty at the household level by moving from subsistence to commercial agriculture. The programme, implemented through the army, distributes free agricultural ‘inputs’ – seeds, seedlings, planting materials and breeding stock (Robert and Katusiimeh Reference Robert and Katusiimeh2018).
Operation Wealth Creation runs under President Museveni’s 2016 manifesto, which had as its slogan, ‘Taking Uganda to Modernity Through Jobs-Creation and Inclusive Development’ (Museveni Reference Museveni2016). Under the National Development Plan II, the agriculture sector goal was to achieve an average growth rate of 6% per year through a focus on increasing production and productivity, access to inputs, access to markets, and strengthening agricultural services institutions (Government of Uganda 2019). The history of livestock programmes in Uganda is one of repeated attempts at improved productivity. Throughout, new animal breeds have been a part of this story, introduced through research and programmes. For example, veterinary scouts in the 1950s were tasked to distribute exotic chickens in communities and to monitor their growth. Introduced as ‘improved’ breeds, these animals have become known locally as enzungu (or ‘exotic’), mirroring terminology used to describe Western foreigners – bazungu – a word conveying different, more delicate, bodies, in this case implying that they require different treatment than local, ‘resistant’ bodies. By extension, the term enzungu, also brings with it ideas of progressive modernity tied to the bazungu, with the expectation of higher productivity that is linked to a better – and wealthier – life.
According to one of the veterinarian key informants, exotic pig and exotic poultry production in Uganda evolved around 1980s, but only began its rapid expansion in the 2000s. Initially introduced into the country for cross breeding purposes due to their fast growth and productivity, large white pigs and layer birds were produced in agricultural institutions and by select farmers. Research continues on modified breeds at the National Animal Genetic Resource Centre and Databank with international partners, for example with the Kuroiler cross-bred chicken, designed to combine the faster and greater growth of a broiler with the ‘resistance’ to diseases of local poultry (Staff Reference Staff2021). By May 2019 the Minister for the Ministry of Agriculture Animal Industries and Fishing (MAAIF) reported that Kuroiler chickens formed the largest number of poultry inputs provided through the Operation Wealth Creation programme in 2018 (Ministry of Finance 2000) although this number is still relatively small at 12,000. Unfortunately, because the planned livestock census in 2020 was delayed due to Covid, the size of the increase in different kinds of animals and farming since the 2008 livestock census is still pending. Interim studies suggest that the increase in livestock will have been substantial and the move towards exotic breeds and intensive approaches will have accelerated especially in peri-urban areas (Dione et al. Reference Dione, Oba, Nsadha, Asmare, Knight-Jones and Doyle2022; Mikecz et al. Reference Mikecz, Pica-Ciamarra, Felis, Nizeyimana, Okello and Brunelli2020).
While livestock farming in cities was previously illegal, although commonly practiced, in 2006 new ordinances under existing by-laws were enacted to provide for the licensing, control and regulation of crop and livestock production activities in the city (Sabiiti and Katongole Reference Sabiiti, Katongole, Maheshwari, Purohit, Malano, Singh and Amerasinghe2014). Official attitudes and support for agricultural development shifted to support and guide urban farmers as well as farmers in rural areas. In addition to efforts to promote commercialization amongst subsistence farmers in rural and urban areas, the Ugandan government created incentives for the expansion of private sector investment in commercial livestock production with an intention for expanding exports. Key informants listed multiple policies to achieve this including land zoning, providing tax holidays, and credit financing, as well as reforming extension services and providing agricultural inputs.
The widespread use of antibiotics has been a relatively recent addition to the agricultural landscape. When first introduced in the mid-20th Century, their use was tightly controlled, along with other veterinary medicines, and they could only be dispensed by veterinary officers. A retired veterinary commissioner explained at interview that “penicillin was used to treat sick exotic chickens, under strict authority” (NK, 65 year old male veterinarian). Unlike elsewhere in the world where antibiotics were being marketed and used for their apparent growth promotion properties from the 1950s (Silbergeld Reference Silbergeld2019), in Uganda their use was restricted to treatment. Antibiotics were mainly imported from Britain and as the 1980s came to an end, more veterinary antibiotics were reported in use – two veterinary key informants related that streptomycin and kanamycin were predominant at this time. This decade saw the advent of the Structural Adjustment Programmes (SAPs) with the promotion of the private sector was attributed to the rise in ‘irrational’ use of antibiotics, when it became possible to purchase antibiotics over the counter from drug stores (Atuhwere Reference Atuhwere2018; Ilukor Reference Ilukor2017). Currently there is no systematic surveillance of antibiotic use in livestock in place beyond aggregates of paper-based national imports and manufacture data in Uganda, which show a narrower range of antibiotics being used than has been found on farm-based research studies (unpublished data). Comparative studies show a much higher frequency of antibiotic use and range of antibiotics in urban than rural livestock farming (Nayiga et al. Reference Nayiga, Kayendeke, Nabirye, Denyer Willis, Chandler and Staedke2020). Efforts to regulate antibiotic use on farms remain ad hoc, although the Ministry of Agriculture, Animal Industry and Fisheries has recently produced guidelines for infection prevention and appropriate antimicrobial use in the animal sector, which asks farmers to “Ensure that antimicrobials are used only with prescription and under supervision of an authorized veterinary professional” (Government of Uganda 2020).
Farming in Wakiso, Kampala
Wakiso is a large and rapidly urbanising district encircling Kampala, the capital city of Uganda (Figure 1). Housing approximately 2 million people, more than double the 2002 population of 900,000 (Uganda Bureau of Statistics 2016), Wakiso’s population have become predominantly urban with 80% classified as urban in 2014 compared with 7.8% in 2002 (Wakiso District Local Government 2017).
Formally part of Mpigi district, Wakiso district came into existence in the year 2000 when three counties of Mpigi District - Busiro, Kyadondo and Entebbe Municipality – were merged with the intention to strengthen local economic competitiveness and enterprise development for sustainable wealth creation, employment and growth into a special status district. The 2016 district development plan includes a watermark on each page with the vision for Wakiso as “A Transformed community from semi peasant to a modern and urbanized district within 30 years” (Wakiso District Local Government 2016). Setting out avenues for investment and enterprise, agriculture makes the top of the list, noting an increasing demand for chicken as a key advantage. District officials have made efforts to attract private investment including in commercial livestock farming, with an eye to the export market. The push for investment in recent years highlighted the potential to expand and overtake existing small-scale farming efforts, for example noting that ‘approximately 49,500 households in Wakiso district keep pigs. However, majority of these are smallholder with an average land holding of ¼ acre in the peri urban areas. The piggery enterprise is predominantly women owned and if practiced on a commercial basis, it can be a massive income generating project for many families.’ (Wakiso District Local Government 2018)(p18). The regulatory environment includes incentives for investments in Wakiso in line with the 2014 Uganda Investment Code and Free zones Act. In the last four years, the research team learned of two foreign owned pig farms – one Chinese and one Korean – that were established to produce high quality meat, targeting niche markets within and outside of the country. However, the majority of farms remain small scale businesses targeting the local market. In addition to private practitioners, there are currently 15 veterinarians and 12 Animal Husbandry Officers employed by the government to cover Wakiso district.
The 115 farms in this study (Figure 1) varied in scale, production systems, and composition of livestock, including pigs only, poultry only, a mix of pigs and poultry, and a few that kept pigs or poultry with another species of food animals. Farms in the survey kept between 11 to 30 pigs and 500 to 5000 birds. Most of the participants (83/115) were farm owners and many were relatively new to the business. The majority were aged 40 – 50 years old and most had attained a secondary school education and had been engaged in other income generating activities prior to coming into farming. Over half had obtained some informal training to identify and manage livestock illnesses. Few had utilized insurance schemes, and additional scoping was required to identify the five farmers who were interviewed about these experiences, who had larger farms – two had 10,000 poultry and one had 5,000 pigs. Antibiotic use was common on the farms visited – 83% of participants said they used antibiotics at least monthly across pig and poultry farms. The antibiotics most commonly used were various brands of tetracycline (76.5% of farmers) – in oral powders, injectables and often unknowingly through consumption of imported concentrate (hybrid) feeds. Antibiotics that are considered critically important antibiotics for human health included dihydrostreptomycin sulphate, reported to be frequently used by 20.9% of farmers (Nayiga et al. Reference Nayiga, Kayendeke, Nabirye, Denyer Willis, Chandler and Staedke2020).
A central observation of this study was the way pigs and poultry were being produced through what can be termed quick farming (Table 1). This form of farming appeared particular to the peri-urban spaces of Wakiso – and differed from rural small holder farm practices in our research in rural Tororo (Nayiga et al. Reference Nayiga, Kayendeke, Nabirye, Denyer Willis, Chandler and Staedke2020). Whilst both study sites faced similar challenges in their farms of security, climate and disease, the ambition in the Wakiso farms was palpably different. Here, farming was situated in a promissory milieu – of optimism for speed and size of animal production through exotic breeds and imported technical fixes. To an extent, the characteristics of farming in Wakiso are shared in common with long standing descriptions of commercialized farming activity elsewhere in the globe, as described in Ruth Harrison’s classic book, “Animal Machines,” and subsequent work in rural sociology and history, agricultural economics and animal ethics: intensification, concentration, specialization, industrialization, productivism and post-productivism (Harrison Reference Harrison1964 ; Bowler Reference Bowler1986; Beus and Dunlap Reference Beus and Dunlap1990). The sociotechnical process of intensification and productivism consists of improving yields by optimising the means of production – through animal confinement, increase of herd size, use of veterinary medicines, and rationalisation of feeding and breeding techniques. This combines with a socioeconomic process of industrialisation, specialisation and concentration that is supported by the development of corporate farming and vertical integration in which one’s own products are created, distributed and sold, with control of downstream industries over upstream actors, as well as tailorisation of labour in which production is broken down into specialised repetitive tasks. The particular observation of quick farming in peri-urban Uganda incorporates the promise of productivity through imported commercial animals and materials with small-scale spaces, limited resources and sparse expertise with the result that the risks inherent in more intensive forms of farming, such as disease, climate and market fluctuations, are much harder to manage. This research describes the ways in which this phenomenon is unfolding in Wakiso through promises to an entrepreneurially alert population who take on these opportunities by navigating new sociotechnical networks and developing new skill sets, combined with a model that saddles both risk and responsibility with those who take these opportunities.
Table 1. Key features of the ‘Quick farming’ phenomenon
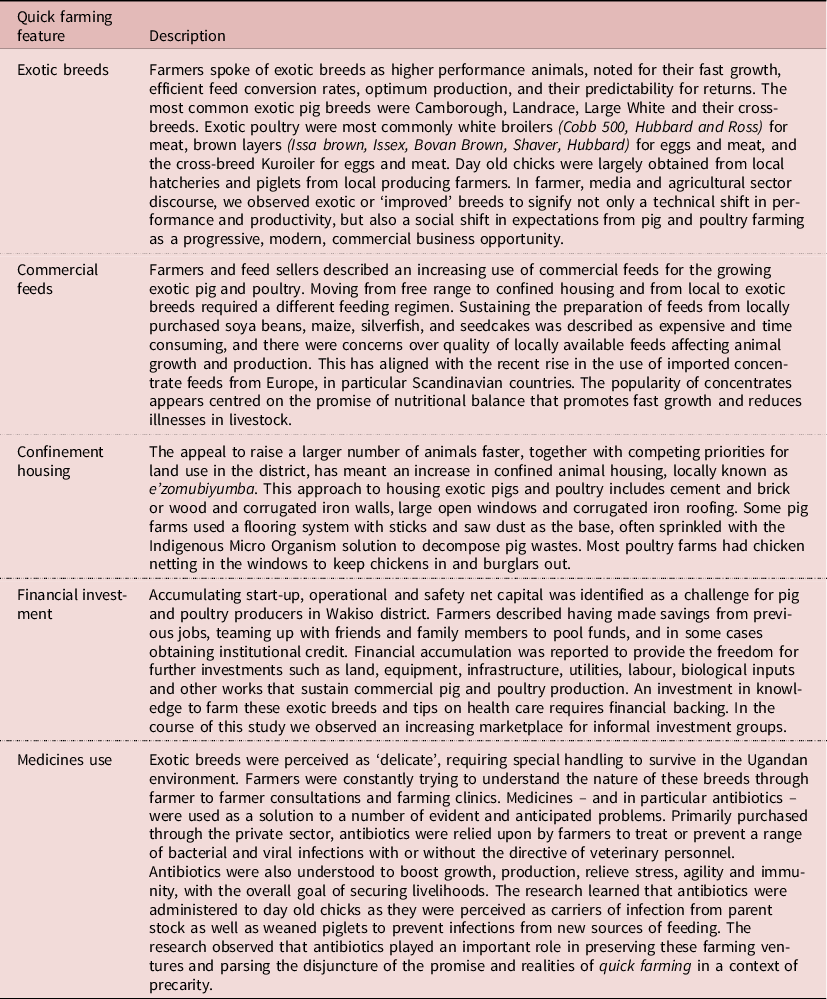
Quick farming, then, emerges ethnographically both as a literal translation of the ability for particular breeds to be nurtured quickly (‘enunda eya mangu’ as farmers described it in Luganda) and as an analytic concept for the wider quest for rapid production described above. It emerges amidst what has elsewhere been termed a promissory assemblage of the urban (Färber Reference Färber, Blok, Farías and Roberts2020). Alexa Färber, an urban anthropologist, draws on Actor Network Theory in her work to observe how for example low-budget housing, consumption and mobility often become unfulfilled promises in the city that are experienced alongside the enduring promises of the city. This promissory assemblage captures the coexistence of the ‘city as a promise’ of modernization and the ‘promised city’ as a desired object and destination for migration. This work draws attention to the connections and contingencies of the social, technical and material dimensions of the hopes and plans for urbanizing, that persist whilst incorporating anticipated and realized failures. It is this affective dimension of the promissory assemblage that resonates strongly with the quick farmers Wakiso, who are living at once with the optimism of the promise and the realities of the risks and challenges to achieve it. Here, antibiotics can be seen as part of this promissory assemblage of peri-urban farming, serving to maintain the optimism that the promise is true and dissociate from the harsh reality of farming conditions. The following sections describe the ways in which the promise is created, the opportunities are taken and the risks are shouldered.
The promise
The first thing that inspired me to put up the pig farm was to eradicate poverty so that I am also not badly off. They have market, and when you want, you get money. I am proud of rearing those pigs because now as I speak I have three degrees in my house. I have paid school fees [for my children] and I am still rearing them because I have two more – one at the university and one in lower secondary. I have got all their fees from pigs. I am proud that piggery helps me a lot. (KA, Male 52 years old, pig farmer for 10 years)
The venture of farming exotic pig and poultry breeds has been portrayed nationally as an opportunity for social mobility; a process whereby people are organised to collectively think and act upon their development which allows people to move ‘up’ social and economic strata. The linking of agriculture to social mobility through sensitisation on modern agriculture and government extension services to train farmers on new technologies is not new, but it appears to have become more widespread with a wider outreach including through social media. Distinguished from traditional knowledges and practices of rural farming, quick farming emerges as an entrepreneurial activity possible in peri-urban spaces. If the vision of Wakiso is to encourage investment in larger scale commercial farms, the promise of quick farming is in practice understood to be realized by anyone, to be taken up as a main or supplemental source of livelihood.
Creating an appetite and opportunities for farming pigs and poultry
My first day at the Harvest Money Expo is the last of the showcase. An entrance fee of 10,000 shillings [approximately 0.3dollars] is charged. Thousands of people are already in attendance, hovering from one stall to the other. Services offered at exhibiting stalls are profit based solutions, including banking, pharmaceuticals, agricultural engineering, improved and fast-growing plants, exotic livestock and poultry, training in modern and urban farming practices, information materials like booklets and audio recordings on farming. It is a busy final day, and attendees are particularly hungry for ‘harvesting’ knowledge from the various stalls. Some observe demonstrations of the magic of new plant and animal production procedures, some ask questions of the exhibitors about how their proposals would work in practice’, others purchase animal breeds, improved plants or seeds, medicines, fertilizers, improved farming equipment such as irrigators, tractors, milk preserving machines, hatcheries and some attentively attend various short trainings while taking notes. The expo appears to be a one-stop centre for a range of different expertise and products. A hallmark of products on display is the label ‘genuine’. The expo also promotes an ongoing competition between farmers practicing improved crop and livestock farming. I am ushered into the popular Dutch Village section of the expo, which promotes Dutch products and agricultural information. Here, many attendees are eagerly listening in to a teaching on pig, poultry, dairy cattle and goat farming. Some Dutch farmers provide training and materials while others are assisted by Ugandans. I learn that masterclasses are offered during the expo to interested people to gain knowledge from Dutch experts on intensive livestock farming, product usability and opportunities to partner with Dutch and other farmers in the value chain for agribusiness. The promise of this expo appears to be to stimulate entrepreneurship for commercial agriculture and provide a discount starter point for farming products. (MK ethnography field notes, February 2019)
Farming ‘clinics’ had become popular spots for promoting exotic livestock farming. Run by public or private service providers including local community-based organizations, expert farmers, and private professionals in production as well as government extension workers, clinics varied between one-off centralized events such as those at the large agricultural exhibitions and on-going initiatives run by private enterprises and through government extension workers. Mass media platforms were also used to communicate modern farming technologies and present market opportunities through media such as television and closed groups like WhatsApp or Facebook platforms. Local media such as print, broadcast and internet browsers regularly advertised pig and poultry as a lucrative enterprise for social and economic mobilization. The language appealed to and enticed the public, presenting golden opportunities to become successful exotic livestock-entrepreneurs, who could plant ‘Seeds of Gold’ and ‘Harvest Money’ (Table 2). This enterprise was often portrayed as an easy-to-start business with trifling finances and a promise of quick returns. Farming exotic livestock was also portrayed as a predictable business model with calculated returns. Echoing the optimism of these ventures, one pig farmer explained at interview how one could grow the enterprise from scratch,
“You can start with a pregnant sow and within three months, you will have between 12 to 15 piglets” (44-year old male farm manager).
Table 2. Creating the appetite for quick farming

Creating demand for pig and poultry production
Pig and poultry farmers who took up modern, high-yielding and space-sensitive production systems positioned themselves to produce for an apparently rapidly growing metropolitan market. The growth in the urban population is often cited as a driver for the demand for meat, but, at the time of this research the market was fluctuating, both before and during the periods marked by Covid-19 lockdowns. There are known periods of the year – in line with religious celebrations for example – that farmers produce for. But, for a more continuous demand amongst local populations, a push for additional festivals and rationales for meat consumption was observed. New events for feasting on animal produce have proliferated – especially through social media – in recent years, for example rolex festivals (a ‘rolex’ is a rolled-up fried egg and chapati, popular as street food in Kampala) and pork parties (Table 2). Such events had been supported to become national fixtures that lent legitimacy as cultural artefacts through their roles as forms of social connection. Brightly designed adverts also entice people to come together and eat chicken buckets. Educational programmes continue, primarily addressing mothers to correct protein deficits as part of concerns over malnutrition (Government of Uganda 2011), although it was not clear that this had changed in pace sufficiently to account for the apparent increased demand for meat by the population.
Taking the opportunity
Mzee Byenkya, a retired public servant in his late 50s is now a poultry farm owner. Without much hope for a paid job, he invested his retirement benefits when he learned of the lucrative business of exotic poultry farming. Equipped with no experience in exotic animal production, a day’s crash course at a famous and successful poultry farm provided a snapshot into establishing a farm. His farm sits on two acres of land stretching inward from a busy marram road; land he purchased five years ago for this purpose. The land was an ancestral home for a previous owner, who sold it and relocated. Its proximity to the road network and other poultry farms meant easy access to raw materials. Initially, the land was a bush of shrubs with tall grasses soaring high. Now, 2,000 brown laying chickens are raised here, in double-story corrugated iron sheet structures. He is always preoccupied with the status of his chickens. With these ‘exotic’ breeds, he knows they are vulnerable to infection as well as to theft. He pays close attention to their behaviour - listening to their chuckling, observing their agility, monitoring their droppings, watching their feeding and egg laying pattern. Changes in any of these signs could mean infection. (MK field notes, July 2018)
With the stage set for high performance animals in marketplaces with keen customers, many have been attracted into quick farming. However, taking up this opportunity required capital and the ability to navigate markets for inputs and outputs.
Capital investment
The participants in this study solicited start-up and maintenance capital in varied ways. Most often start-up capital derived from personal savings from salary accumulation, pension funds, and support from friends and family members. Tales circulated of strategic businessfolk who acquired and invested large sums of bank loaned money into predicted seasons timed with lucrative pig and poultry yields. Start-up capital was essential to establish pre-operating conditions such as the physical infrastructure (land, electricity, equipment, plumbing, structures including buildings, fencing, stores and security) as well as operating expenses such as purchase of feeds, vaccines and medicines, cleaning materials, staff salaries and meals. Most farmers had pivoted existing compound spaces or land into this enterprise. Farmers could often sustain a season of production with start-up capital, but most relied on borrowing additional funds from friends and family to keep farms operational. Money lenders – often referred to as ‘fire extinguishers’ – where the last resort for sourcing operational funds. Some individuals took advantage of recent agricultural loans policy whereby the government supported private financial institutions to lend individuals at an interest rate of 12.5%, which was low when compared to the rate offered by general commercial loans (as high as 20% per year). Whilst the increasingly common informal savings groups (Food and Agriculture Organization 2009b; Nannozi Reference Nannozi2019) were recognised to fund pig and poultry enterprises, farms known to have been set up by savings group members were difficult to find. The common modes of capital accumulation enabling investments in pig and poultry production meant farmers would start small, with only some choosing to expand the business over time.
Individuals involved with pig farming had to financially invest sizable portions of their start- up capital to purchase special exotic breeds of pigs that were advertised to produce a large litter size of between 10 to 18 piglets and yet produce a thin layer of fat with more lean meat. According to one key informant (EN, a 42 year old woman who had worked as a pig farmer and private veterinarian for 16 years), she imported her first pure exotic sow from South Africa at $4000 and sold piglets at $145 each. Although all farmers would have liked to acquire pure breeds of animals for their clean production line, this was im possible due to the high procurement and maintenance costs. Most resorted to purchasing locally bred animals whose pedigree is generally uncertain, which in turn compromised the product quality and income levels.
Navigating markets
Once capital had been secured to initiate the farming enterprise, for those new to farming – or at least to the farming of exotic animals – individuals were then faced with navigating marketplaces of products and expertise to ensure the animals thrived as well as to sell their grown pigs, piglets, poultry or eggs. Knowledge of products – medicines, disinfectants, feeds and breeds – was primarily linked to media marketing information and interaction with informal animal healthcare actors – or ‘paraprofessionals’ (Arvidsson et al. Reference Arvidsson, Klara Fischer, Sternberg-Lewerin and Chenais2022) with varied levels of training. Access was often through walk-in outlets where farmers paid in cash or on credit. Although their qualifications were often in question, these paraprofessionals were drawn upon to advise on illnesses and administer medicines – often injectable antibiotics – that farmers were less familiar with or unable to manage. They could also advise on products considered of good quality including medicines, feeds and young chicks and piglets. Farmers built up relationships with them to learn illness management skills on-site, and obtain over-the-phone consultations. Most farmers purchased and stored a variety of antibiotic types at the farm premises.
In addition, the services of market brokers were vital to the success of a farmer. Market brokers were the link between pig and poultry farms and the available market for their products. They collected products from most farms at a negotiated and agreed upon farm gate price. After sales, the broker charged 10% of the total profits and returned the remaining profits to the farmer. Farmers were required to produce ‘high quality’ animal products, but also with desirable attributes that would eventually be sold at an affordable price. For example, the demand for yellow-yolked eggs had pushed farmers to purchase additional supplementary diets such as Carophyll – a popular colouring agent used as a feed additive – to promote yellowing of the yolk. Carophyll supplement was often purchased in kilograms each costing about $5. Each kilo would mix about 100 kilograms of feed, making the ingredient quite costly. With time, Carophyll was incorporated into feed concentrates – adding to the many ingredients that led to its marketing as a ‘complete meal’.
Risky realities
Pig and poultry production was described as a journey of twists and turns. Even when running smoothly, just as one production cycle ended, the need to acquire capital to enable the second production cycle commenced. Depending on the profits made from the sales of farm products, individual farmers were able to restock varied quantities of pigs or poultry for the next production cycle. But often, farming was not smooth. In practice, farmers described numerous risks beyond financial inputs to achieving the promise of quick farming. These included the delicate nature of the animals, disease outbreaks, theft, fluctuating markets and unpredictable climatic changes. Observations showed the intricacy of the animals’ care by farmers and farm workers (described elsewhere – Denyer Willis et al forthcoming) in response to their delicate nature, as described by this farmer who had been in the business for over a decade and had 100 pigs and 10,000 poultry,
Poultry, they are very delicate. If you starve a chicken for an hour it’s supposed to eat at 8:00 and you give it at 9:00 o’clock you might lose quite a number of them. They scratch themselves, they cry, they get stressed very fast unlike piggery. A pig can survive on water for the whole day until you find food but a chicken no. Yes, you have lost it, if it has not died then you might see issues like in a week it will not give you eggs because the other day you starved it. Yes, not like the pigs. (JK, a 45 year old woman who had farmed pigs and poultry for 16 years)
Disease outbreak prevention and management required a range of interventions, and farmers related different management, vaccination and biosecurity arrangements in line with their available resources. For all, however, fear of an outbreak was an important spectre to their business, as described by this young farmer,
I had an outbreak of swine fever, that’s why I haven’t recouped my capital yet because I think I lost about 95% of my herd. At that time I had around two hundred pigs but most of them died and I was only left with about fifteen piglets. That swine fever case almost ran me to zero because everything was lost. If I had sold my stock I would make over sixty million or seventy million plus [around 16-18,000USD]. Imagine feeding thirty pigs to maturity, they produce the first round and produce the second time and then they start dying! (Pig Farmer, (EN, a 30 year old man who had farmed pigs for 4 years)
To keep the animals safe – from disease and theft – was a substantially greater investment in time and care than was widely acknowledged. Through repeated cycles of production, farmers related trying different approaches to boost the resilience of the animals, the security of the farm, the attentiveness and reliability of the farm workers. However, the risks encountered were multiple and uncertain, rendering quick farming a business with razor thin margins and through which antibiotics emerged as an essential protector of investment, as described by this poultry farmer,
[Without antibiotics, you would be] risking the business because we keep these birds as a part of getting money and it is a business where we put our money. So we put more to boost our income. It is like leaving your food outside for the robbers to come and take, then you are not serious! We have to minimise losses and keep everything so that you can get profit outcome of the business. So you must care otherwise without it anything can come like bacterial diseases … If they come they come wash away all the birds in the house. If you have one thousand you remain with one bird. (PP 43-year old female poultry farm owner, well educated including a diploma)
The disease risks for pig and poultry production was understood to be particularly acute for exotic pig and poultry breeds, said to be vulnerable and able to succumb to poor growth and infections. ‘These ones are like white people, if you treat them the local way they may … cause a loss in business,’ as one farmer explained (OT, 51 year old male farm manager). The major disease risks were viral and bacterial infections that caused massive drops in daily production and often resulted in massive deaths of livestock. This reflects evidence of high rates of disease. A study of Wakiso poultry farms in 2018 found Escherichia coli and Salmonella in over a third of birds, and resistance to commonly used antibiotics was high – 100% of E.coli isolates were resistant to tetracycline, 80% resistant to trimethoprim sulphamethoxazole and 64% to ampicillin (Kabaalu Reference Kabaalu2019).
Antibiotics as Protection
From farmers, veterinary officers and agricultural workers, the concern was repeated that the increase in commercially orientated meat production was associated with an increase in the use of antibiotic medicines in pig and poultry farming in Wakiso and beyond. Here, farmers were observed to use antibiotics as a form of protection of their livelihoods, alongside other protections where affordable. Thus, antibiotics assumed roles beyond providing care; they assumed a safety net function, enabling production during ‘peak’ seasons and keeping farms ‘afloat’ in low seasons. One older man, who had farmed poultry for the past two years, described how antibiotics tend to increase eggs production in layers.
The problem is that you cannot put your money on the test. You cannot put millions of money on test and you say ‘me on my farm I don’t use antibiotics’. Yet the people around you, on the other farms are using antibiotics. You could be putting your business at risk! (In-depth interview with EK, 50 year old male poultry farmer)
Few farmers had used insurance, and those who did – except for one, a larger scale farm with strong investment and organisation – found it an expensive and suboptimal experience, with difficulties in registering each animal, maintaining the expected security and biosecurity standards, and recovering funds for losses. Farmers saw little point in paying for insurance when in practice they would be taking the risks themselves.
Despite the numerous risks and losses, farmers kept on with their efforts. The concept of failure was not common in peoples’ narratives; rather there was an acceptance of the waves of profits and loss, even if the magnitude of loss could at times be catastrophic. Farmers described ‘lessons’ rather than ‘failures’, embarking on another production cycle enthusiastic to implement new measures to avoid losses the next time. Here, the promise as an assemblage contains elements that ensure its renewal – the entrepreneurial imaginary of quick farming as a narrative precludes ‘failure,’ pointing to the significance of antibiotics and other technical components such as breeds and feeds as bridging the disjuncture between dreams and reality.
Discussion
This paper presents the biosocial phenomenon of quick farming in Uganda, which interconnects microbial, economic, social and political concerns, highlighting how antibiotics are relied upon to achieve people’s aspirations for entrepreneurship as part of a nationwide vision of modernity and progress through agriculture. Although this research included only 115 farms, which may not be representative of the district’s farms as a whole – especially with larger commercial farms being harder to access, the wide range of interviews with stakeholders together with the feedback discussions with participants and other actors, solidified our interpretation of this phenomenon.
The observation of the expansion of quick farming as part of a peri-urban promissory assemblage (Färber Reference Färber, Blok, Farías and Roberts2020) underscores the significance of an affective orientation in the pursuit of particular livelihoods. The flexibility described by the farmers in finding capital, navigating markets for inputs and outputs, and for continuing to try and to tweak their methods is central to entrepreneurialism, as described by Carla Freeman; the ability to respond to ever-changing circumstances, to re-tool and re-train. Furthermore, entrepreneurialism can be understood as a way of being in the world, beyond a mechanism for income generation (Freeman Reference Freeman2014). This has significance for how the quick farming model of livestock rearing is understood in context. It illustrates the consequences of an ideology and experimentalism of behavioural economics that has underscored many agricultural development programmes in which the poor are cast as informal and backwards, in need of modernization to turn profits, to become entrepreneurial rational actors who are less afraid to take the necessary risks to release themselves from poverty (Berndt Reference Berndt2015).
This paper’s findings of the growth of small-scale commercially-influenced peri-urban farming is not unique to the study setting. Others have recently described similar set-ups for poultry in Benin and Burkina Faso (Butcher, Cañada, and Sariola Reference Butcher, Cañada and Sariola2021), as well as in aquaculture in Bangladesh (Hinchliffe et al. Reference Hinchliffe, Butcher, Rahman, Guilder, Tyler and Verner-Jeffreys2021) and across multiple settings with dairy farming (Groot and van’t Hooft Reference Groot and van’t Hooft2016) – in each case achieving this with reliance on antibiotics as part of the farming model. Concerns over the impacts of an ‘urbanizing livestock revolution’ include environmental and public health threats, with changed land-use, animal density and frequency of human-animal and domesticated-livestock interactions (Latino, Pica-Ciamarra, and Wisser Reference Latino, Pica-Ciamarra and Wisser2020). The relatively high rates of antibiotic use on small scale farms, including in urban settings, has now been described elsewhere in Uganda (Mikecz et al. Reference Mikecz, Pica-Ciamarra, Felis, Nizeyimana, Okello and Brunelli2020) as across many African countries, with a systematic review showing 100% of farms using antibiotics in studies from Tanzania, Cameroon, Zambia, Ghana and Egypt (Kimera et al. Reference Kimera, Mshana, Rweyemamu, Mboera and Matee2020), as well as high levels of multidrug resistant isolates, at 100% in studies on farms in South Africa, Zimbabwe and Tunisia. The challenge of drug resistance is amplified for farms that rely on antibiotics – affecting further their ability to maintain livelihoods. Furthermore, the significance for human health has become a widely accepted concern. How best to address this concern in practice remains a challenge, especially at the interface of policies pulling in opposite directions – crudely, urbanizing agriculture under the guise of poverty alleviation and nutritional sufficiency, versus improving animal husbandry under the threat of antimicrobial resistance amongst other health and environmental concerns. The One Health framework offers an opportunity to address such tensions across sectors, although currently this ideology sits more in principle than in practice given the organization of activity and funding within sectors.
To address the high levels of antibiotic use on the kinds of farms in this study, we concur with the consensus that additional expertise would be valuable – there is a strong desire to understand best practices for food inputs, biosecurity, vaccination, farm management and medication. While a majority of the training or information received occurs through unregulated private actors and businesses, this desire risks being met with advice that would conflict with public health alert best practices. There are few veterinarians and animal husbandry officers operating close to farmers’ everyday practices, overshadowed by the volume of informal paraprofessionals and businesses offering advice. Expanding the professional expertise available to farmers could be an important step and should be distinguished from general attempts to raise awareness of antimicrobial resistance. Efforts to address antimicrobial resistance have often taken an awareness raising stance in both humans and animal health (Musoke et al. Reference Musoke, Kitutu, Mugisha, Saba, Brandish, Ikhile, Kajumbula, Kizito, Lubega, Niyongabo, Ng, O’Driscoll, Hobbs, Winter and Gibson2020; Adesokan et al. Reference Adesokan, Akanbi, Akanbi and Obaweda2015; Mikecz et al. Reference Mikecz, Pica-Ciamarra, Felis, Nizeyimana, Okello and Brunelli2020). The limitations of expecting a change in awareness to lead to a reduction in antibiotic use has been described (Pearson and Chandler Reference Pearson and Chandler2019). The expansion of the Farmer Field School model presents and opportunity to build new networks and expertise, supporting farmers to find alternative forms of protection for their animals, through technical apparatus and up-skilling in biosecurity (Food and Agriculture Organization 2009a; Forster and Charnoz Reference Forster and Charnoz2013; Figué and Desvaux Reference Figué, Desvaux, Morand, Lefait-Robin and Apiwathnasorn2015) addressing both on-farm and off-farm factors (Caudell et al. Reference Caudell, Stella Kiambi, Eric Koka, Kimani and Dorado-Garcia2022). The research presented in this paper suggests it is the larger and more organized farms who appear to be better able to incorporate antibiotic stewardship.
Antibiotic use as a buffer for farmers from volatile markets, diseases and unreliable climate provides one of several examples of the ways these medicines are used as technical fixes for problems typically not cast as medical. This exemplifies processes of pharmaceuticalisation, which, together with capitalization and globalization, can be understood to co-create new political economies (Gaudilliere and Sunder Rajan Reference Gaudilliere and Sunder Rajan2021). It reinforces and expands understanding of how antibiotics have come to substitute for care, to stand in for hygiene and sanitation infrastructure, and to ‘fix’ inequalities (Denyer Willis and Chandler Reference Denyer Willis and Chandler2019). Where antibiotics here are not only enabling productivity but providing a layer of protection for farmers, one must then ask what it would take to reduce reliance on antimicrobials for this protection? The research presented in this paper points to the significance of safety nets, and it proposes that insurance and compensation policies could stand as a counter measure to offset the risks currently mitigated through antibiotic use. The findings of this research suggest that such schemes must provide timely responses to claims and ensure that joining requirements such as veterinary checks and security operations do not exclude farmers with minimal capital.
Conclusion
This paper describes the rise of quick farming in peri-urban Wakiso district in Uganda. The latest in a series of agricultural commercialisation initiatives, promising a path to a modern and prosperous future, this research illustrates how this formulation relies upon antibiotics to protect farmer investments and the lives of their families. With rising concerns about antibiotic resistance these farmers will be targeted for educational interventions, aiming to ‘improve’ their practices. Unless other approaches are introduced to protect those engaged with the quick farming enterprise, which is poised to grow in line with the rapid expansion envisioned for the agricultural sector, farmers are likely to continue using antibiotics as part of a promissory assemblage of peri-urban farming in a context of precarity.
Availability of data and materials
The dataset supporting the conclusions of this article is available in the United Kingdom Data Service: https://reshare.ukdataservice.ac.uk/854612/.
Acknowledgements
We wish to thank the district veterinary and production office teams, extension animal health workers and farmers of Wakiso district who provided support and guidance during the data collection process. We are grateful Dr Lawrence Mugisha of the College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, who supported the project from inception.
Authors’ contributions
Author contributions to this paper are as follows: C Chandler and S Nayiga conceptualized the study and design; data collection was led by M. Kayendeke with input from S Nayiga, C Nabirye and L Denyer Willis; data coding, analysis and interpretation of results was done by M. Kayendeke and supported by C. Chandler and L. Denyer Willis; preparation of manuscript was led by M. Kayendeke and C. Chandler; critical revision was performed by C. Chandler, N. Fortané, L. Denyer, C. Nabirye and S. Staedke. All authors reviewed the drafts and approved the final manuscript.
Funding
Funding was provided through the Antimicrobials In Society (AMIS) grant, awarded to the London School of Hygiene & Tropical Medicine by the Economic and Social Research Council (ESRC) on behalf of the Antimicrobial Resistance Cross Council Initiative supported by the seven Research Councils UK (RCUK) in partnership with other funders [ES/P008100/1].
Ethics approval and consent to participate
The research protocol received ethical approval from the School of Biomedical Sciences Research and Ethics Committee (SBS REC), Makerere University College of Health Sciences; Uganda National Council for Science and Technology (UNCST) and the London School of Hygiene & Tropical Medicine Observation Ethics Committee. Written consent for inclusion in the study was provided by all participants.





