In the U.S., approximately 11% of infants are born small for gestational age (SGA), defined as birth weight below the 10th percentile for gestational age-specific birth weight (McCowan, et al., Reference McCowan, Figueras and Anderson2018). SGA birth is typically the result of fetal growth problems during pregnancy, where the fetus does not receive essential nutrients and oxygen required for healthy fetal development (Osuchukwu & Reed, Reference Osuchukwu and Reed2022). Infants born SGA have an increased risk of neonatal morbidity and mortality (Finken, et al., Reference Finken, van der Steen, Smeets, Walenkamp, de Bruin, Hokken-Koelega and Wit2018). Research has suggested that substance use, exposure to infectious diseases, exposure to pollution, and maternal socioeconomic status are associated with SGA birth (Finken, et al., Reference Finken, van der Steen, Smeets, Walenkamp, de Bruin, Hokken-Koelega and Wit2018). However, one possible risk factor that is less understood is early menarche of the mothers.
Menarche is an important event occurring during puberty that signifies the beginning of a woman’s reproductive capacity. Early menarche, defined as menarche before age 12, is linked to numerous adverse chronic health effects (Li, et al., Reference Li, Song, Shen, Liu, Zheng, Zhang, Li, Xia, Lu, Zhang, Zhou, Cao, Wang and Xu2017). Previous research has also demonstrated that early menarche is associated with statistically significant increased odds of adverse reproductive health outcomes such as gestational diabetes (Shen, et al., Reference Shen, Hu, D. Taylor, Kan and Xu2016), preterm birth (Li, et al., Reference Li, Song, Shen, Liu, Zheng, Zhang, Li, Xia, Lu, Zhang, Zhou, Cao, Wang and Xu2017), and low birth weight (Xu, et al., Reference Xu, Jarvelin, Lu, Xu and Rimpela1995). However, the early menarche-SGA birth association is under researched and limited to studies conducted outside of the U.S. In fact, to our knowledge only one study conducted in the last 20 years has examined this relationship, and results suggested no association between early menarche and SGA (menarche at age nine, RR: 0.99, 95% CI: 0.62-1.59; menarche at age 10, RR: 0.87, 95% CI: 0.73-1.05; menarche age 11, RR: 1.04, 95% CI: 0.92-1.18) (Kanno et al., Reference Kanno, Kyozuka, Murata, Isogami, Yamaguchi, Fukuda, Yasuda, Suzuki, Sato, Ogata, Shinoki, Hosoya, Yasumura, Hashimoto, Nishigori and Fujimori2022). As this study was also conducted in Japan, it is unclear if the findings also extend to U.S. women. Regardless, menarche is associated with a gradual increase in estradiol levels (Valeggia & Núñez-de la Mora, Reference Valeggia and Núñez-de la Mora2015), and research has demonstrated that women who experience early menarche have higher estradiol levels than women who experience early menarche later in adolescence (Emaus, et al., Reference Emaus, Espetvedt, Veierød, Ballard-Barbash, Furberg, Ellison, Jasienska, Hjartåker and Thune2008; Vikho & Apeter, Vihko & Apter, Reference Vihko and Apter1984). Furthermore, increased estradiol levels are associated with higher inflammation, and high inflammation levels is associated with an increased risk of SGA birth (Hu et al., Reference Hu, Feng, Lin, Zhong, Zhu, Lv, Lv, Meng, Zhang, Lu, Jin, Sheng, Xu and Huang2014; Svensson, et al., Reference Svensson, Just, Fleisch, Sanders, Tamayo-Ortiz, Baccarelli, Wright, Téllez-Rojo, Wright and Burris2019). Thus, the early menarche-SGA association may be facilitated through this biologic mechanism.
The purpose of this study was to examine the early menarche-SGA association using a population-based sample of U.S. women. Data were retrieved from the 2011-2013, 2013-2015, and 2015-2017 National Survey of Family Growth (NSFG). The NSFG is a cross-sectional, continuous survey used to collect and analyze information on family planning and various reproductive health-related areas (Shen et al., Reference Shen, Hu, D. Taylor, Kan and Xu2016). The combined sample size consisted of 16,854 women (average response rate=69.3%). In this study, women were excluded for the following reasons: reported age of menarche was >19 (n=62) or <8 (n=12), age at first birth <18 or >35 or missing (n=4,030) or missing demographic information (n=652). Additionally, women were excluded if the index pregnancy/birth resulted in a stillbirth (n=164), birthweight was unknown (n=107), did not occur between 22-45 weeks gestation (n=6,178), or was a multiple birth (n=82). Thus, our final analytic sample included 5,567 women with singleton births.
The main exposure was early menarche. Women self-reported age at menarche during an in-person interview conducted by trained NSFG staff. Consistent with previous studies, women were considered exposed if menarche occurred <12 years old (Kanno et al., Reference Kanno, Kyozuka, Murata, Isogami, Yamaguchi, Fukuda, Yasuda, Suzuki, Sato, Ogata, Shinoki, Hosoya, Yasumura, Hashimoto, Nishigori and Fujimori2022). The outcome of interest was SGA birth. Women self-reported the gestational age and birth weight of their firstborn child during the in-person interview. Infants born below the tenth percentile for gestational age were considered SGA (Schlaudecker, et al., Reference Schlaudecker, Munoz, Bardají, Boghossian, Khalil, Mousa, Nesin, Nisar, Pool, Spiegel, Tapia, Kochhar and Black2017).
Maternal age at first birth, education level, race/ethnicity, marital status, and annual household income were considered as possible confounders (McCowan, et al., Reference McCowan, Figueras and Anderson2018). Logistic regression was used to obtain unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) of the early menarche-SGA association. A multivariate model was created by first including all potential confounders, and backward elimination was used to retain only those variables with p<0.20 (Budtz–Jørgensen, et al., Reference Budtz–Jørgensen, Keiding, Grandjean and Weihe2006). Data were analyzed using SAS-callable SUDAAN to account for the complex sampling design used by NSFG.
Approximately 4% of mothers had an SGA infant and 23% of mothers reported early menarche (Table 1). Consistent with prior research, marital status, race/ethnicity, and lower annual household income were associated with increased odds of SGA birth (Finken et al., Reference Finken, van der Steen, Smeets, Walenkamp, de Bruin, Hokken-Koelega and Wit2018; McCowan et al., Reference McCowan, Figueras and Anderson2018). Prior to adjustment, mothers with early menarche had 12% greater odds of having an SGA infant (OR: 1.12, 95% CI: 0.76, 1.65). After adjustment for annual household income, maternal age, and maternal race/ethnicity, the association was attenuated (OR: 1.03, 95% CI: 0.70, 1.53).
Table 1. Study sample characteristics 2011-2017 NSFG (N = 5,567); unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) of the associations between select characteristics and small for gestational age birth
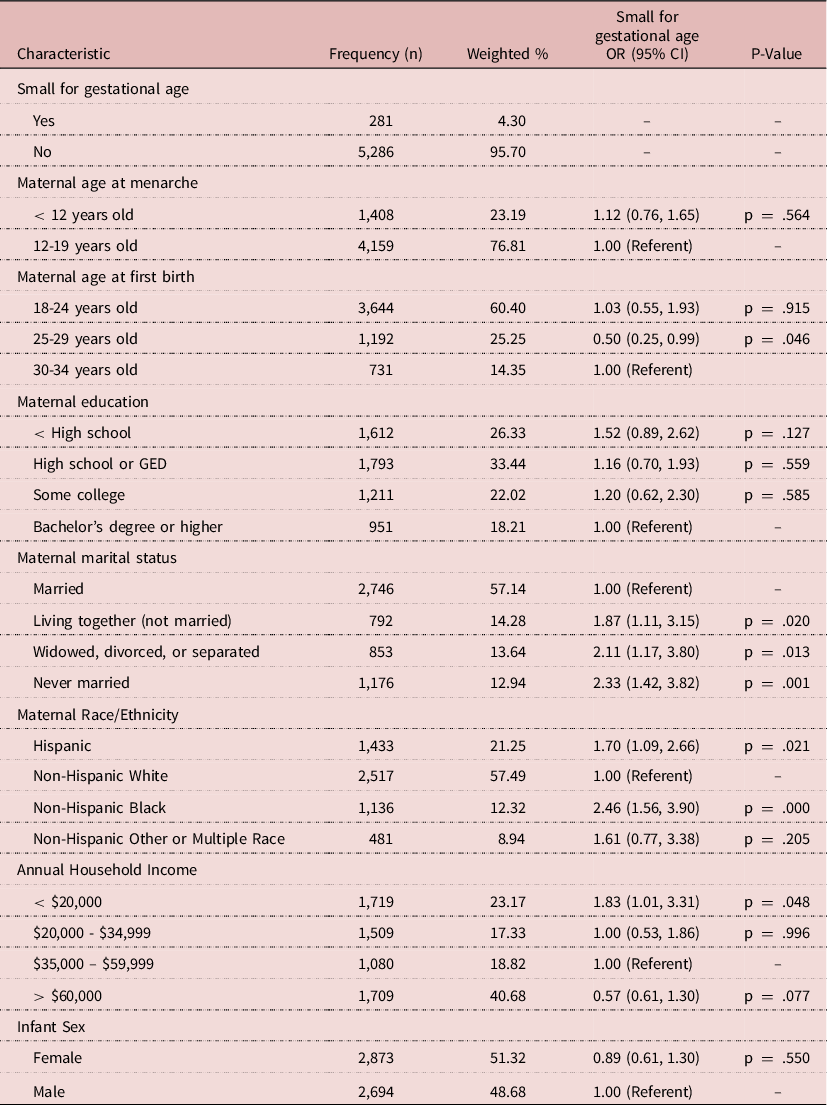
In this population-based study of women who recently had a live singleton birth, we found no strong association between early menarche and SGA birth after adjustment. To our knowledge, no previous U.S. study has examined the relationship between early menarche and SGA birth. Our results are similar in magnitude and congruent with one recent study conducted in Japan whose results also suggested that there was no statistically significant association between early menarche and SGA birth (Kanno et al., Reference Kanno, Kyozuka, Murata, Isogami, Yamaguchi, Fukuda, Yasuda, Suzuki, Sato, Ogata, Shinoki, Hosoya, Yasumura, Hashimoto, Nishigori and Fujimori2022).
Limitations of this study include non-differential misclassification of exposure and outcome, as women self-reported age at menarche and gestational age and may not accurately remember this information. However, previous studies have demonstrated strong validity of self-reported age at menarche (Cooper et al., Reference Cooper, Blell, Hardy, Black, Pollard, Wadsworth, Pearce and Kuh2006; Dorn et al., Reference Dorn, Sontag-Padilla, Pabst, Tissot and Susman2013; Koprowski, Coats, & Bernstein, Reference Koprowski, Coates and Bernstein2001; Lundblad & Jacobsen, Reference Lundblad and Jacobsen2017; Must et al., Reference Must, Phillips, Naumova, Blum, Harris, Dawson-Hughes and Rand2002) in women decades older than women in our sample, as well as strong validity of self-reported gestational age (Chin, et al., Reference Chin, Baird, McConnaughey, Weinberg, Wilcox and Jukic2017). Additionally, the NSFG survey questions do not include maternal behaviors during pregnancy; thus, it was not possible to control for other potential confounders.
Despite these limitations, our study had many strengths. First, the use of trained NSFG interviewers and the strong response rate minimized the possibilities of information bias and selection bias, respectively. Furthermore, to our knowledge only one study conducted in the last 20 years has examined early menarche and SGA birth, and it was conducted outside the U.S. As NSFG data are designed to be nationally representative, our results are likely generalizable to U.S. women aged 18-34.
In summary, this study fills a notable gap in the literature regarding early menarche and SGA birth. Additional research is needed in diverse populations to further address the early menarche-SGA birth association, and to evaluate if age at menarche is associated with other adverse birth outcomes.
Funding
This research received no specific grant from any funding agency, commercial entity or not-for-profit organization.
Conflict of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. As this was a secondary data analysis of routinely collected, de-identified data, additional ethical approval from the local Institutional Review Board was not necessary as it was not considered to be human research.



