Published online by Cambridge University Press: 27 March 2009
In 1914 the result of a study of the evaporation of water from soil was published by B. A. Keen, who distinguished two factors governing the rate of evaporation. One factor was that the available surface from which evaporation takes place decreases as the moisture content decreases. Keen deduced an equation that expressed the operation of this factor, viz.
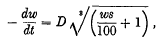
in which D is a constant, w = percentage of water by weight and s = the real specific gravity of the soil. He showed that this equation agreed with his experimental results with a reasonable degree of accuracy.
page 121 note 1 Journ. Agric. Sci. 6 (1914), 456–75.Google Scholar
page 121 note 2 Really ![]() where p 1 = v.p. of the evaporating liquid and p 2 = the partial v.p. of that liquid in the atmosphere. This obviously reduces to
where p 1 = v.p. of the evaporating liquid and p 2 = the partial v.p. of that liquid in the atmosphere. This obviously reduces to ![]() when the drying atmosphere is of zero humidity.
when the drying atmosphere is of zero humidity.
page 122 note 1 Text. World Record, 36 (1908), 219–26.Google Scholar
page 122 note 2 Trans. Amer. Soc. Mech. Eng. 39 (1918), 1073–1128.Google Scholar
page 123 note 1 Proc Roy. Soc. 78 (A) (1906), 412–29.Google Scholar
page 123 note 2 Zeit.f. Elektrochem. 17 (1911), 800–805Google Scholar; Koll. Chem. Bethefte, 9 (1917–1918), 1–182.Google Scholar
page 123 note 3 Koll. Zeit. 17 (1915), 78–104.Google Scholar
page 124 note 1 Soil Soi. 11 (1921), 409–34.Google Scholar
page 126 note 1 Roy. Soc. Proc. 95 (A) (1923).Google Scholar
page 128 note 1 U.S. Bureau of Soils, Bul. 10 (1897).Google Scholar
page 128 note 2 Ibid.Bul. 38.
page 128 note 3 Soil Sci. 10 (1920), 103–26; 11 (1921), 215–32.Google Scholar
page 128 note 4 Buckingham, , loc. cit.Google Scholar; Gardner, W., loc. cit.Google Scholar
page 131 note 1 [Note added Feb. 22nd, 1923.Google Scholar Since this waa written the work has been extended in various directions and to other materials. The results indicate that the curvature in the evaporation curve for clay soil is not the simple shrinkage effect described above. This point will be discussed in detail in a later paper and may necessitate a modification in some respects of the point of view developed in this section.—E. A. F.]
page 132 note 1 The first weighings were taken at about 57% water content after the first fine cracks had already appeared. Although these increased in number and width they produced no appreciable variation in the rate of evaporation as long as free water was evaporating. Thus the experimental time curve between 57% and 35% water showed no indication of curvature, the rate curve for this region being straight and horizontal. The rate of evaporation over this region, however, was not quite identical with that from the silty soil which showed no signs of cracking. Thus the silty soil lost 114 e.g. of water in 2·471 hours (in the region covered by the straight portion of the experimental time curve) as against 114·93 c.g. in the same time for quartz sand and 118·07 c.g. for the Rothamsted subsoil. The values should be identical in all three cases. The sand lost 0·815% more water than the silt and this may be regarded probably as an indication of the order of magnitude of the experimental error inherent in the method. The Rothamsted subsoil lost 3·57% too much water and the excess may be attributed to the slightly increased evaporating surface in this case due to the formation of the cracks.
page 132 note 2 Lewis, W. K., Journ. Ind. and Eng. Chem. 13 (1921), 427–32CrossRefGoogle Scholar; Geller, R. F., Journ. Amer. Cer. Soc. 4 (1921), 282–7.CrossRefGoogle Scholar
page 133 note 1 Sci. Abs. (Physics), 22 (1919), 171.Google Scholar
page 133 note 2 Phil. Mag. 34 (1917), 308–21.Google Scholar
page 134 note 1 See experimental part.
page 135 note 1 The writer is indebted to Mr N. M. Comber for this sample and for details of its mechanical analysis.
page 136 note 1 Loc. cit.
page 140 note 1 Loc. cit.
page 140 note 2 Trans. Far, Soc. 17 (1922), 244–8, 288–94.Google Scholar
page 141 note 1 Proteins and the. Theory of Colloidal Behaviour, J. Loeb (McGraw-Hill Pub. Co.), 1922.Google Scholar