Published online by Cambridge University Press: 02 May 2022
The goal was to study imprinting effects on birth weight (BW), weaning weight (WW), six months weight (W6), pre- and post-weaning growth rate (GRa and GRb), and pre- and post-weaning Kleiber ratio (KRa and KRb) in Baluchi and Makuie sheep breeds. Analyses were done in a two steps process. In the first step, each trait was analysed with a series of 12 univariate animal models, including different combinations of direct and maternal effects and the best model was selected by the AIC criterion. In the second step, three new models were fitted by adding either maternal imprinting effect, paternal imprinting effect, or both of them, respectively, to the best model already selected in the first step, and changes in AIC were monitored. (Co)variances between traits were estimated using bivariate analyses. In Baluchi sheep, for BW, WW and W6, estimates of maternal imprinting heritability (${\boldsymbol h}_{{\boldsymbol mi}}^2$ ) were 0.12 ± 0.02, 0.08 ± 0.01 and 0.09 ± 0.01, respectively. In Makuie sheep, paternal imprinting heritability (${\boldsymbol h}_{{\boldsymbol pi}}^2$
) were 0.12 ± 0.02, 0.08 ± 0.01 and 0.09 ± 0.01, respectively. In Makuie sheep, paternal imprinting heritability (${\boldsymbol h}_{{\boldsymbol pi}}^2$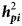 ) for BW, WW, W6, GRb and KRb were 0.17 ± 0.05, 0.8 ± 0.05, 0.38 ± 0.10, 0.71 ± 0.15 and 0.65 ± 0.13, respectively. In Baluchi sheep, strong maternal imprinting correlations were observed between BW and WW (0.93 ± 0.05), BW and W6 (0.80 ± 0.07) and WW and W6 (0.91 ± 0.02). In Makuie sheep, paternal imprinting correlations ranged from −0.97 ± 0.33 (BW-GRb) to 0.99 ± 0.47 (GRb-KRb). It was concluded that imprinting effects need to be included in the statistical model to increase the accuracy of genetic evaluation. However, to have accurate and reliable estimates of imprinting effects, the availability of large data sets and deep pedigrees are necessary.
) for BW, WW, W6, GRb and KRb were 0.17 ± 0.05, 0.8 ± 0.05, 0.38 ± 0.10, 0.71 ± 0.15 and 0.65 ± 0.13, respectively. In Baluchi sheep, strong maternal imprinting correlations were observed between BW and WW (0.93 ± 0.05), BW and W6 (0.80 ± 0.07) and WW and W6 (0.91 ± 0.02). In Makuie sheep, paternal imprinting correlations ranged from −0.97 ± 0.33 (BW-GRb) to 0.99 ± 0.47 (GRb-KRb). It was concluded that imprinting effects need to be included in the statistical model to increase the accuracy of genetic evaluation. However, to have accurate and reliable estimates of imprinting effects, the availability of large data sets and deep pedigrees are necessary.