INTRODUCTION
The global warming caused by anthropogenic greenhouse gas production is of increasing concern. Ruminants have a considerable impact on global warming since they contribute one-sixth of the total atmospheric methane (CH4). In addition, per molecule, CH4 is 23 times more potent as a greenhouse gas than carbon dioxide (CO2), even though it has a shorter half-life (Houghton et al. Reference Houghton, Ding, Griggs, Noguer, van der Linden, Dai, Maskell and Johnson2001). Thus, efforts to mitigate CH4 emissions from ruminants are critical given that CH4 concentration is increasing at a faster rate than CO2.
In tropical agriculture, poor quality of feed coupled with low feed conversion efficiency leads to high levels of CH4 emission per kg of food produced from ruminants. Researchers are actively engaged in evaluating the potential of secondary plant constituents as natural means of modifying ruminal fermentation. Various secondary compounds such as tannins and saponins are present in plants to protect them from bacteria, fungi, herbivorous insects and vertebrates. Tannins are prevalent in many tropical fodder plants, and when consumed at high concentration can cause adverse effects on the rumen microbial population and animal health. At lower doses, tannins reduce the release of ammonia (Makkar Reference Makkar, Gill, Owen, Pollot and Lawrence1993) and inhibit methanogenesis (Bhatta et al. Reference Bhatta, Vaithiyanathan, Shinde and Jakhmola2005, Reference Bhatta, Enishi, Takusari, Higuchi, Nonaka and Kurihara2008), but the relative effect of polyphenols on methanogenesis has received attention only recently.
Previous in vitro rumen fermentation studies demonstrated that the reduction in methanogenesis by tannins was due to their direct effects on methanogenic archaea as well as indirect effects on protozoa (Bhatta et al. Reference Bhatta, Uyeno, Tajima, Takenaka, Yabumoto, Nonaka, Enishi and Kurihara2009). However, very few studies have been conducted on the effect of feeding tannin on in vivo rumen methanogenesis in animals, such as those of Puchala et al. (Reference Puchala, Min, Goetsch and Sahlu2005) and Carulla et al. (Reference Carulla, Kreuzer, Machmuller and Hess2005) who recorded lower methanogenesis in goats and sheep, respectively, fed condensed tannin (CT). However, Beauchemin et al. (Reference Beauchemin, McGinn, Martinez and McAllister2007) failed to reduce enteric methane emissions from growing cattle fed with 0·02 of the dietary dry matter (DM) as quebracho tannin extract. Generally, CH4 suppression recorded in vivo was lower compared to that of in vitro conditions (Fievez et al. Reference Fievez, Dohme, Danneels, Raes and Demeyer2003). The novelty of the current approach compared with earlier studies is that a combination of hydrolysable tannin (HT) and CT was used as the tannin source, since tannin may not exist as pure CT or HT in nature. Systematic attempts were made to evaluate the effects of feeding commercially available tannins to goats at two concentrations on rumen fermentation, methanogenesis and energy partitioning. Firstly, tannin inclusion levels in the current study were much lower than in previous studies (McSweeny et al. Reference McSweeny, Palmer, McNeill and Krause2001; Roth et al. Reference Roth, Steingass, Drochner and Pen2001). Secondly, goats are reported to be more resistant to tannins than sheep or cattle (Silanikove et al. Reference Silanikove, Gilboa, Perevolotsky and Nitsan1996); therefore, one aim was to determine whether reduced methanogenesis could be observed even in goats.
MATERIALS AND METHODS
Experimental design, diets and animals
A study involving adult male Japanese goats (34 ± 1·5 kg live weight (LW)) was carried out over three sequential experimental periods to evaluate the effects of control, low-tannin and high-tannin dietary treatments in a switch-over design. The control diet was the basal diet, which consisted (as feed basis) of timothy (Phleum pratense) hay (0·65), crushed maize (0·20) and soybean meal (0·15), whereas the low-tannin diet consisted (as feed basis) of 0·975 of the basal diet and 0·025 of a commercial tannins source compound. The high-tannin diet included 0·95 of the basal diet and 0·05 of the tannin source. The source of tannins was a commercial product (from Mimosa) available as fine powder with a light brown colour (Kawamura and Co. Ltd, Asakusabashi, Taito-ku, Tokyo, Japan). The product contained 37 g CT/kg DM and 76 g HT/kg DM.
Goats were fed the control diet for a 2-week period (in individual cages) and then they were moved to the energy metabolism laboratory, where the study was carried out. Each experimental period consisted of 21 days, which included 14 days acclimatization and a 7-day metabolism/balance measurement period, at the end of which the goats were moved to respiration chambers for a 3-day gaseous exchange measurement.
The animals were fed once a day and any refused feed was collected and measured daily (at 09·30 h, after c. 24 h feed offer). Feeding level was set at 1·2 times the maintenance metabolizable energy (ME) requirements, estimated according to Itoh et al. (Reference Itoh, Haryu, Tano and Iwasaki1978). All goats received humane care as outlined in the ‘Guide for the care and use of experimental animals’ at National Institute of Livestock and Grassland Science, Tsukuba, Japan.
Balance measurement and collection of rumen samples
Over a 7-day period, feed intake was accurately measured by collecting representative samples of feed on offer and refusals. These samples were oven-dried at 60 °C for 48 h and ground to pass through a 1 mm sieve for analysis. Over the same period, total faecal and urinary outputs were collected daily (before feeding). Faeces were collected using trays and all the daily outputs were retained for processing. Urine was collected in buckets containing 30 ml of 2·4 m H2SO4 and 0·10 of the daily output was sampled. Faeces and urine samples were stored frozen until the collection period was completed when the daily samples were pooled within individual animals, mixed thoroughly and sub-sampled. For additional analysis, faecal samples were oven dried at 60 °C for 48 h and ground to pass through a 1 mm sieve, while 20 ml urine was weighed in a polyethylene film and freeze-dried for determination of gross energy (GE; Itoh & Tano Reference Itoh and Tano1977).
At the end of the balance period ruminal contents were collected from each goat using stomach tubing. This was carried out 4 h post-feeding. The collected samples were immediately measured for pH using a pH meter and then stored frozen (–30 °C) for analysis of NH3–N and volatile fatty acid (VFA) concentrations.
Gaseous measurements
Emissions of CO2 and CH4 and consumption of O2 were measured using open circuit respiration chambers of the energy metabolism laboratory at the National Institute of Livestock and Grassland Science (NILGS, Tsukuba), which consists of four individually controlled respiration chambers. The animals were housed at 09·00 h and remained there for three consecutive days. The chambers were opened once a day for feeding and cleaning, and collection of faeces and urine. In brief, the chambers were equipped with a computer-controlled air-handling system to provide the following conditions: temperature 15–35 °C), humidity 30–80% and air flow rate through the apparatus of 30–500 litres/min. During measurements, temperature and relative humidity in the chambers were maintained at 20 °C and 60%, respectively.
Analytical methods
For the estimation of tannin, a 0·1 g sample of tannin source was extracted with 10 ml of 70% (v/v) aqueous acetone in a 50 ml stoppered Erlenmeyer flask for 20 h at room temperature. After centrifugation at 2795 g for 15 min, the supernatant was made up to 10 ml by the addition of methanol and used for the determination of CT using the butanol HCl protocol in the presence of iron (Makkar Reference Makkar1984) and HT using rhodanine reagent (Inoue & Hagerman Reference Inoue and Hagerman1988). The CT was expressed as leucocyanidin equivalent and HT as gallotannin (Makkar Reference Makkar1984).
VFAs were determined (Tajima et al. Reference Tajima, Nonaka, Higuchi, Takusari, Kurihara, Takenaka, Mitsumori, Kajikawa and Aminov2007) by gas chromatography (6890 series gas chromatograph with a flame-ionization detector, Hewlett-Packard, Wilmington, DE, USA) on a glass column with 5% Thermon 1000 and 0·5% H3PO4 on 80/100 mesh Chromosorb W (Wako Pure Chemical Ltd., Osaka, Japan). The NH3–N was determined colorimetrically (Tajima et al. Reference Tajima, Nonaka, Higuchi, Takusari, Kurihara, Takenaka, Mitsumori, Kajikawa and Aminov2007) with a Technicon Auto analyser II (Shimadzu, Tokyo, Japan).
Tannin sample, diet and faecal samples were analysed for DM, crude protein (CP; N × 6·25), ash (AOAC 1990), neutral detergent fibre (NDF; Van Soest et al. Reference van Soest, Robertson and Lewis1991) and acid detergent fibre (ADF; Robertson & Van Soest Reference Robertson, van Soest, James and Theander1981). The NDF was analysed in diet samples without sodium sulphite (but with sodium sulphite in tannin samples) and with the use of a heat-stable amylase. Both NDF and ADF were expressed with residual ash. The GE content of samples was determined in an adiabatic bomb calorimeter (CA-4PJ, Shimadzu, Kyoto, Japan).
The energy values of the feed, faeces and urine samples collected were used to calculate energy balance. The GE contents of feed, faeces and urine were determined using the values for the heat of combustion in a bomb calorimeter (CA-4PJ, Shimadzu, Kyoto, Japan). Digestible energy (DE) was calculated as the difference between GE intake and faecal energy (FE) output. ME was calculated as the difference between DE intake and the output of both urinary energy (UE) and CH4. Methane gas volume was converted to energy using the conversion factor 39·539 (kJ/l). Heat production (HP; kJ) was calculated by the equation: HP (kJ/day) = 16·175 × O2 (litres/day) + 5·021 × CO2 (litres/day) − 2·167 × CH4 (litres/day) − 5·987 × urinary nitrogen (g/day) (Brouwer Reference Brouwer and Blaxter1965).
Statistical analysis
The independence and homogeneity of variance of the three diets over time was tested by a time series plot of residuals (Box–Ljung statistical analysis). The autocorrelation with lags 1 and 2 was also performed for the diets. The data were analysed by PROC GLM using SAS/STAT Version 9.1 (SAS Institute 2004) by F-test and indicated by their P-value. The general model used was Y ij=μ+α i+β j+αβ ij+ε ij, in which Y ij was the dependent variable, μ was the least square mean, α i was the effect of tannin, β j was the effect of animal, αβ ij was the effect of interaction of tannin and animal and ε ij was the residual error. If the treatment effect was significant in the model, differences among treatments were determined using Tukey's multiple comparison procedure. Least squares means, standard errors of the means and P-value in the model were reported and effects were considered significant at probability of P < 0·05.
RESULTS
Chemical composition of the tannin source and diets
The tannin source was a commercially available, light brown, fine powder and contained (g/kg DM) 213 CP, 38·6 NDF and 18·5 ADF with a GE content of 20·0 MJ/kg DM. The CT and HT contents were 36·7 and 76·2 g/kg DM, respectively (Table 1).
Table 1. Composition (g/kg DM) of tannin source and experimental diets
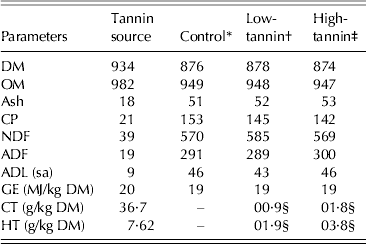
CT, condensed tannin as leucocyanidin equivalent; HT, hydrolysable tannin as gallotannin; ADL, acid detergent fibre; (sa), sulphuric acid.
* Control: consisted of 0·65 timothy hay, 0·20 crushed maize and 0·15 soybean meal.
† Low-tannin: contained 0·975 of control diet + 0·025 tannin.
‡ High-tannin: contained 0·95 of control diet + 0·05 tannin.
§ Calculated values.
Total tannin concentrations were 2·8 (0·9 CT + 1·9 HT) and 5·7 (1·8 CT + 3·8 HT) g/kg DM for low- and high-tannin diets, respectively. The CP contents of control, low- and high-tannin diets were 153, 145 and 142 g/kg DM, with NDF of 570, 585 and 569, respectively and the GE contents were 18·9, 19·0 and 19·0 MJ/kg DM, respectively. There were no differences (P > 0·05) in fibre fractions of the three diets.
Feed intake and digestibility
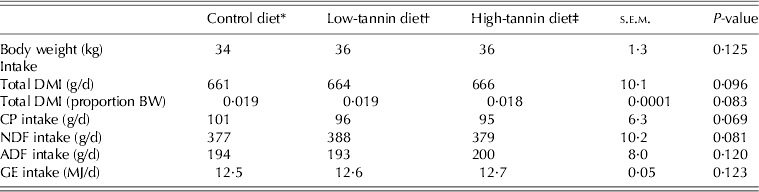
The dietary treatments had no effect (P > 0·05) on feed DM or GE intakes, or on intakes of CP or fibre fractions (Table 2). However, there were diet effects on digestibilities of feed constituents (Table 3). In general, except for CP, the control and low-tannin diets did not differ (P > 0·05) in feed digestibility, but digestibility values for the high-tannin diet were much lower (P < 0·05) than those of control and low-tannin diets. Intakes of the digestible fractions of diets reflected differences in their digestibilities (Table 3).
Table 2. Intake by goats fed diets containing two concentrations of tannins

* Control: consisted of 0·65 timothy hay, 0·20 crushed maize and 0·15 soybean meal.
† Low-tannin: contained 0·975 of control diet + 0·025 tannin.
‡ High-tannin: contained 0·95 of control diet + 0·05 tannin.
DMI, dry matter intake; CP, crude protein; NDF, neutral detergent fibre; ADF, acid detergent fibre; GE, gross energy.
Table 3. Digestibilities and digestible intake (g/d) of dietary components in goats fed diets containing two concentrations of tannins

* Control: consisted of 0·65 timothy hay, 0·20 crushed maize and 0·15 soybean meal.
† Low-tannin: contained 0·975 of control diet + 0·025 tannin.
‡ High-tannin: contained 0·95 of control diet + 0·05 tannin.
NDF, neutral detergent fibre; ADF, acid detergent fibre; DMI, dry matter intake; OMI, organic matter intake; CPI, crude protein intake; NDFI, neutral detergent fibre intake.
Table 4 shows nitrogen (N) intake and partition. N intakes in tannin-containing diets were slightly lower than those in the control diet. Dietary treatments had effects on N partition. Faecal N loss increased (P < 0·05) with the inclusion of tannins in the diet, whereas urinary N decreased (P < 0·05). The amount of digested and retained N per unit of intake was highest in goats fed the control diet. The total loss of N as a proportion of N intake was lowest in the control diet, followed by high- and low-tannin diets (Table 4).
Table 4. Nitrogen utilization in goats fed diets containing two concentrations of tannins

* Control: consisted of 0·65 timothy hay, 0·20 crushed maize and 0·15 soybean meal.
† Low-tannin: contained 0·975 of control diet + 0·025 tannin.
‡ High-tannin: contained 0·95 of control diet + 0·05 tannin.
Rumen fermentation products
Table 5 presents ruminal pH, and concentrations of NH3–N and VFA for each of the dietary treatments. The pH values were similar across the dietary treatments (P > 0·05), whereas NH3–N concentrations decreased (P < 0·05) with increasing tannin concentration in the diet. The ruminal concentrations of total VFA, acetic acid (C2), propionic acid (C3), butyric acid (C4), valeric acid (C5) and iso-acids (isobutyric + isovaleric acids, iC4 + iC5) corresponding to the high-tannin diet were the lowest compared to those in control and low-tannin diets, whereas concentrations of C3 and C5 were higher and lower, respectively in the low-tannin diet than control diet. The lowest C2:C3 was found in the low-tannin diet, whereas the highest value was for the high-tannin diet.
Table 5. Rumen pH, NH3–N (mg/l) and VFA concentrations (mM) in goats fed diets containing two concentrations of tannins
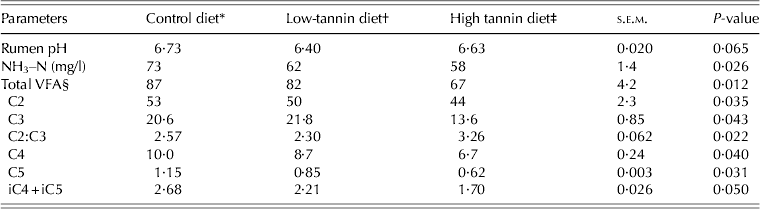
* Control: consisted of 0·65 timothy hay, 0·20 crushed maize and 0·15 soybean meal.
† Low-tannin: contained 0·975 of control diet + 0·025 tannin.
‡ High-tannin: contained 0·95 of control diet + 0·05 tannin.
§ C2, acetic acid; C3, propionic acid; C4, butyric acid; C5, valeric acid; iC4 + iC5, isobutyric + isovaleric acids.
Energy partition and methane emission
Table 6 shows the feed energy partition and CH4 emission. As previously stated, GE intakes were similar across the experimental diets. FE output increased (P < 0·05) with increasing concentrations of tannins in diet (9 and 40% more than the control) and consequently goats fed the high-tannin diet had the lowest DE intakes (2 and 13% less in low- and high-tannin diets than the control). However, the high-tannin diet was associated with the lowest UE output (11 and 16% less in low- and high-tannin diets, respectively, than the control) (P < 0·05). Methane emissions, expressed either on absolute basis (g/d, energy/d) or per unit of live weight (LW), digestible organic matter intake (DOMI) or GE intake were lowest (P < 0·05) for the high-tannin diet, whereas the highest (P < 0·05) emissions were associated with the control diet (11 and 23% less in low- and high-tannin diets than control) (Table 6). For example, the methane conversion ratio (MCR) for the high-tannin diet was 0·75 of that of the control diet (Table 6). Nevertheless, ME intake on the high-tannin diet was lower than for the control and low-tannin diet.
Table 6. Energy balance in goats fed diets containing two concentrations of tannins

* Control: consisted of 0·65 timothy hay, 0·20 crushed maize and 0·15 soybean meal.
† Low-tannin: contained 0·975 of control diet + 0·025 tannin.
‡ High-tannin: contained 0·95 of control diet + 0·05 tannin.
GE, gross energy; DE, digestible energy (GE–FE); MCR, methane conversion ratio; ME, metabolizable energy.
Autocorrelation at lag 1 and lag 2 concentrations for the three diets were not statistically significant. The Box–Ljung statistical method (Ljung & Box Reference Ljung and Box1978) to test the independence of serial measurements based on the three diets indicated that they were not statistically significant (P > 0·05), thereby confirming independence and homogeneity of variance between times, i.e. CH4 measured from successive diets from each goat were independent.
Energy digestibility and ME/GE values were higher in control and low-tannin diets when compared to that of high-tannin diet. The loss of DE as CH4 was affected (P > 0·05), while the proportional energy loss in urine was not affected (P > 0·05) by the presence of tannin in the diet. The proportion of ME lost as HP was higher (P < 0·05) for low- and high-tannin diets than for the control. A similar trend was also observed in retained energy (RE)/ME, with lowest value seen in goats fed the high-tannin diet.
DISCUSSION
In the present study, intakes of DM, GE and major dietary constituents were not affected by the inclusion of tannins in the diet. Generally, tannins adversely affect DM intake because of their astringency and reduced DM digestion in the rumen, which reduces passage of digesta through the gut leading to gut fill (Silanikove et al. Reference Silanikove, Gilboa, Perevolotsky and Nitsan1996). The absence of negative effects on intake in the current study can be attributed to three factors: first, the diet used was a total mixed ration and tannins were present as fine powder, and because of uniform distribution with soybean meal and maize particles the palatability may not have been affected. Secondly, the highest inclusion of tannin source was only 50 g/kg DM of the total diet, thereby achieving a net tannin concentration of 6 g/kg DM in the high-tannin diet, whereas it was only 1·9 g/day or 2·8 g/kg DM in the low-tannin diet. And thirdly, the feeding level was 1·2 × maintenance v. ad libitum feeding. Therefore, the concentration of tannin was not high enough to have an adverse effect on intake. It has been reported that <40 g tannin/kg DM diet is generally not detrimental to intake in ruminants (Barry et al. Reference Barry, Manley and Duncan1986).
A known consequence of the presence of tannins in feed is reduced ruminal fibre degradation because tannins prevent the adherence of microbes to food particles or directly inhibit microbial cellulolytic activity (Leinmuller & Menke Reference Leinmüller and Menke1990). A significant decrease in DM and organic matter (OM) digestibilities in goats and sheep fed chestnut-tannin-treated hay was recorded in a study by Zimmer & Cordesse (Reference Zimmer and Cordesse1996), but the tannin dose was very high (80 g/kg DM). In the current study, tannin at 2·8 g/kg DM diet (low-tannin) did not adversely affect the digestibility of dietary components (except CP), but the high-tannin diet did affect feed digestibility and intakes of digestible fractions of the diet. Tannins have the capacity to bind cell walls and cell solubles, reducing microbial fermentation (Kumar & Vaithiyanathan Reference Kumar and Vaithiyanathan1990).
In tannin-containing diets protein degradation was markedly reduced, resulting in lower NH3–N concentration, since tannins have been shown to inhibit microbial deaminase and urease activities (Makkar Reference Makkar, Gill, Owen, Pollot and Lawrence1993). Lower ruminal NH3–N concentration with CT-containing forage Lotus corniculatus compared to CT-free forage has been reported in ewes (Min et al. Reference Min, McNabb, Kemp and Barry1998) and in vitro (Williams et al. Reference Williams, Eun, MacAdam, Young, Fellner and Min2011). Increased ruminal NH3–N concentration results from bacterial protein engulfed by protozoa and subsequently recycled via the ruminal NH3–N pool (Jouany Reference Jouany1994). Reduced (P<0·05) ruminal NH3–N and protein degradation observed in tannin-fed goats may be explained by the associated inhibition of the diet and bacteria-degrading activities of the protozoa (Jouany Reference Jouany1994).
The iso-acids are derived from amino acid catabolism in the rumen (Mackie & White Reference Mackie and White1990). In the current study, the observed reductions in NH3–N and iso-acids suggest reduced amino acid deamination (Table 5). Inhibition of amino acid deamination has practical implications because it may increase ruminal outflow of amino acids and improve the efficiency of N use in the rumen (Van Nevel & Demeyer Reference van Nevel, Demeyer and Hobson1988).
Recently, it has been shown (Bhatta et al. Reference Bhatta, Uyeno, Tajima, Takenaka, Yabumoto, Nonaka, Enishi and Kurihara2009) that CT possesses both anti-methanogenic and anti-protozoal activities in vitro. The source of tannin used in the current study was the same as that of the previous in vitro study (Bhatta et al. Reference Bhatta, Uyeno, Tajima, Takenaka, Yabumoto, Nonaka, Enishi and Kurihara2009). The proportion of DE lost as CH4 in the low-tannin diet without reduction in the digestibility of cell wall fraction is attributable to the reduced ruminal methanogenic archaea and protozoa population and activity in goats, which confirms in vitro findings (Bhatta et al. Reference Bhatta, Uyeno, Tajima, Takenaka, Yabumoto, Nonaka, Enishi and Kurihara2009). This was also strongly supported by the ratio of acetic to propionic acid in the rumen, which was reduced from 2·57 in control diet to 2·30 in low-tannin diet, reflecting a higher propionate concentration, a preferred hydrogen sink product. Methane production is mainly a function of cell wall digestibility, hence the lowest CH4 output in goats fed high-tannin diet may be explained by the fact that reduced OM digestibility recorded in these animals resulted in reduced rumen VFA, acetate concentration and CH4 output.
Recently, a meta-analysis of the relationship between dietary tannin level and methane formation (Jayanegara et al. Reference Jayanegara, Leiber and Kreuzer2011) clearly demonstrated that tannins reduce methane emission in ruminants. The current results are in agreement with an earlier study (Waghorn Reference Waghorn and Rode1996) in which it was reported that sheep fed Lotus pedunculatus (80 g CT/kg DM) yielded less CH4 than when fed on perennial ryegrass (Lolium perenne) or grazing on lucerne (Medicago sativa) pasture (3·9, 6·2 and 5·7% GE intake, respectively). Similar responses were also observed when dairy cows were fed silages of L. pedunculatus or perennial rye grass (Woodward et al. Reference Woodward, Waghorn, Ulyatt and Lassey2001). In addition, other in vitro reports have also found depression of CH4 production with other CT-containing plant species such as Leucena lecucocephala (Huang et al. Reference Huang, Liang, Tan, Yahya and Ho2011) and sainfoin (Onobrychis viciafolia) (McMahon et al. Reference McMahon, Majak, McAllister, Hall, Jones, Popp and Cheng1999). In another study (Clark et al. Reference Clark, De Klein and Newton2001), in which diets differing in protein source (soybean meal, pea or rapeseed meal) were supplemented with very high proportion of tannin-rich extracts from chestnut and mimosa (HT) or quebracho (CT), chestnut tannin was very effective in reducing CH4 production. Huang et al. (Reference Huang, Liang, Tan, Yahya and Ho2011) recorded that the proportion of in vitro CH4 in total gas decreased with increasing molecular weight (MW) of tannin, concluding that MW had a direct impact on CH4 production and it was more pronounced for CT with higher MW. Recently, Pellikaan et al. (Reference Pellikaan, Stringano, Leenaars, Bongers, van Laar-van Schuppen, Plant and Mueller-Harvey2011) observed a reduction in CH4 production by valonea and myrabolan tannins and their structural characterization revealed that chebulic-type ellagitannins reduce CH4 production. Woodward et al. (Reference Woodward, Waghorn, Ulyatt and Lassey2001) reported that CH4 emission relative to digestible DMI was decreased by 24–29% when CT-containing forage L. pedunculatus was fed compared with rye grass or lucerne in sheep. A similar decrease (23%) in CH4 emission relative to DMI was also observed by the same authors when cows were fed L. corniculatus silage compared with rye grass.
In the current study, the salient finding was that of reduction (P<0·05) in CH4 emission by goats fed low-tannin diet compared to control in spite of similar fibre digestibilities (NDF and ADF) between the two groups. This supports the previous finding of Bhatta et al. (Reference Bhatta, Uyeno, Tajima, Takenaka, Yabumoto, Nonaka, Enishi and Kurihara2009) that at lower concentrations of tannin, CH4 suppression was primarily due to direct effect of reduction in number of methanogenic archaea and/or indirect effect on reduced protozoal number. Methanogens have ecto- and endosymbiotic relationships with protozoa (Finlay et al. Reference Finlay, Esteban, Clarke, Williams, Embley and Hirt1994). However, at higher concentrations (high-tannin diet) of tannin, reduction in methanogenesis was due to the combined effect of reduced archaea/protozoa and OM (fibre) digestibility. This was also reported in a recent review by Jayanegara et al. (Reference Jayanegara, Leiber and Kreuzer2011)
The presence of CT in the diet shifts the N excretion from urinary N to faecal N (Bhatta et al. Reference Bhatta, Krishnamoorthy and Mohammed2000). The proportions of intake N lost in urine and faeces were affected by the diet, but total N loss was the lowest for animals fed control diet. The higher faecal N and lower urinary N loss per unit of N intake in goats fed high-tannin diet showed a higher absorption of N that resulted in higher retained N/unit N intake and higher retained N/unit N digested in control when compared with low- and high-tannin diets. Shifting of N excretion from urine to faeces is preferable for prevention of environmental pollution, because volatilization of ammonia from urinary N is much higher than from faecal N (Castillo et al. Reference Castillo, Kebreab, Beever and France2000).
Methane output expressed per unit of DOMI, one of the suggested parameters for CH4 emission inventory, was reduced by 9 and 11% for low- and high-tannin diets, respectively, and when emissions were expressed on an energy basis (CH4 energy/GE intake) the corresponding reductions in emission were even higher (12 and 25% for low- and high-tannin diets, respectively). However, the lower ME intake observed in the high-tannin diet can be attributable to the energy lost as FE, despite lowered UE and CH4 energy in the high-tannin diet. Partition of energy reflected that tannin increases energy loss as FE and reduced UE and CH4 energy.
The HP was similar among the groups, indicating that plant tannins in the diet had no effect on the total HP in goats. The RE reflected similar trend as that of ME since there was no difference in HP among the groups. The CO2 production was similar between the control and the low-tannin diet, but lower (P < 0·05) in the high-tannin diet. This was primarily due to reduced OM fermentation in goats fed high-tannin diet.
CONCLUSIONS
The current results unequivocally demonstrated that tannins (CT + HT) at low concentration (2·8 g/kg DM) reduce CH4 emission in goats without adversely affecting the digestibility of dietary components. However, at higher concentration of tannin (5·7 g/kg DM) intake, CH4 reduction was also attributed to reduced OM fermentation. These results suggest the potential for tannins to minimize CH4 emission from ruminants and to develop practical means to exploit the use of tree leaves containing appreciable amount of tannins.
The study was supported by the Global Environment Research Fund from the Ministry of Environment and the research fund from the Ministry of Agriculture, Forestry and Fisheries of Japan. R Bhatta is grateful to the Japan Society for the Promotion of Science for awarding the JSPS postdoctoral research fellowship under which this work was undertaken. The technical assistance of Ms Nirasawa and Ms Shimada and the help of the staff of the animal shed for the care and management of the experimental animals are acknowledged.