Published online by Cambridge University Press: 15 February 2024
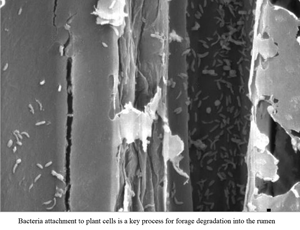
Three male sheep were fed, throughout three experimental periods, with either only forage, only total mixed ration (TMR) or a mixed diet (TMR + forage). The rich-fibre ingredients of each diet were incubated daily in situ for three days and the ruminal pH was measured every 2 h during the last day of each experimental period. Rumen pH decreased at increased proportion of TMR in diet (P < 0.05). The dry matter (DM) degradability of the grass forage was higher (P < 0.05) in animals receiving only forage than in those receiving the mixed diet whereas the DM degradability of the corn silage was higher (P < 0.05) in animals receiving the mixed diet than in those receiving only TMR. The level of microbial adherence in residues of grass forage was higher (P < 0.05) in animals fed with only forage than in those fed with the mixed diet and, the level of microbial adherence in residue of corn silage was higher (P < 0.05) in animals receiving the mixed diet than in those receiving TMR. The carboxymethylcellulase activity in residues of grass forage was higher (P < 0.05) in sheep fed the mixed diet whereas not significant effect of diet type was observed for this variable in residues of corn silage. In conclusion, increased inclusion of TMR in sheep diet showed a negative impact on microbial adherence and forage degradability in situ, an effect mediated by changes in rumen pH which was not compensated by increased fibrolytic activity.