Introduction
Copper (Cu) is an indispensable and crucial trace mineral for livestock (Olukosi et al., Reference Olukosi, Van Kuijk and Han2019). Cu is involved in several physiological processes that influence growth development, stimulate the immune system against infections, red blood cell production, repair damaged tissues, and eliminate reactive oxygen species which enhances antioxidant capacity (Mroczek-Sosnowska et al., Reference Mroczek-Sosnowska, Tukasiewicz, Wunk and Sawosa2016; Hill and Shannon, Reference Hill and Shannon2019; Abdullah et al., Reference Abdullah, Masood, Zaneb, Rabban, Akba and Kuthu2022). These improvements are linked to Cús role in the development and activity of certain metalloenzymes (such as superoxide dismutase, ceruloplasmin or cytochrome oxidase), which are involved in several metabolism processes including cellular respiration, red blood formation or glucose and cholesterol metabolism (Scott et al., Reference Scott, Vadalasetty, Chwalibog and Sawosz2018). For these reasons, poultry diets frequently incorporate Cu supplementation (Olukosi et al., Reference Olukosi, Van Kuijk and Han2019; El Sabry et al., Reference El Sabry, Stino and El-Ghany2021).
The National Research Council (1994) suggests incorporating Cu into the diet of laying quails at a rate of 5 mg/kg. However, standard poultry feeding practices include high doses of Cu (150–200 mg/kg) to improve certain aspects of avian production such as performance (Olgun and Aygun, Reference Olgun and Aygun2017; Wu et al., Reference Wu, Dai, Hua, Hu, Wang and Wen2019; Deo et al., Reference Deo, Biswas, Sharma and Tiwari2023), eggshell quality (Kaya et al., Reference Kaya, Kaya, Macit and Kaynar2018; Cufadar et al., Reference Cufadar, Curabay, Gökmen, Bahtiyarca and Sevim2022), and antioxidant capacity (Kara et al., Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021; Abdullah et al., Reference Abdullah, Masood, Zaneb, Rabban, Akba and Kuthu2022; Ali, Reference Ali2018). Despite the improvements observed, an excess of dietary Cu may potentially hinder the bioavailability of specific minerals like iron or calcium and could pose a hazard to the environment due to increased excretion in faeces and urine (Kara et al., Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021; Ibrahim et al., Reference Ibrahim, EL-Gendi, Nihad, Okasha and El-Attrouny2022). Some countries have already limited the dietary content of Cu in species such as pigs to levels of 75–100 mg/kg of feed (Kara et al., Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021). Therefore, it is necessary to find safe sources and doses of Cu that improve animal production without compromising the environment (Lin et al., Reference Lin, Guo, Liu, Wang, Su, Yu and He2020).
Cu supplements are generally added to the avian diet such as ionic forms. Ionic sources such as Cu sulphate (CuSO4), are extremely sensitive in contact with water and can be degraded readily both in the feed and in the intestinal tract. This process decreases its absorption in the small intestine due to its interaction with other nutrients (including vitamins, enzymes, or fats), and consequently increases its excretion into the environment (Olukosi et al., Reference Olukosi, Van Kuijk and Han2019; Deo et al., Reference Deo, Biswas, Sharma and Tiwari2023). Hence, the use of new mineral additives has attracted the attention of the poultry industry in recent years, as an alternative to traditional trace mineral sources (Olukosi et al., Reference Olukosi, Kujik and Han2018; Lin et al., Reference Lin, Guo, Liu, Wang, Su, Yu and He2020). Cu hydroxychloride is an organic salt, which is chemically more stable and bioavailable than Cu sulphate, which leads to enhanced absorption and consequently a reduction in excretion into the environment (Olukosi et al., Reference Olukosi, Van Kuijk and Han2019).
Quail production has increased in recent decades, mainly due to their ease of management and high production rates (Sarmiento-García et al., Reference Sarmiento-Garcia, Olgun, Kılınç, Sevim and Gökmen2023). Nonetheless, unlike conventional avian species, fewer investigations have been carried out concerning the nutritional aspects of laying quails and are generally based on the nutritional values reported by the National Research Council (1994) 30 years ago (Olgun et al., Reference Olgun, Gül, Kılınç, Yıldız, Çolak and Sarmiento-García2022). To the best of our knowledge, the number of studies on the Cu requirements of laying quails is quite limited, and even more so when alternative sources such as hydroxychloride are involved. Therefore, the objective of this study is to investigate how different levels of hydroxychloride copper in laying quail diets affect various parameters, such as performance, eggshell quality, yolk antioxidant capacity, tibia characteristics, and mineral excretion.
Materials and methods
Ethical approval
The following study was performed using farm animals; for this reason, there were no special requirements for keeping experimental animals. However, it is noteworthy that the criteria outlined in the European Animal Protection Policy (EPCEU, 2010) were strictly adhered to during the entire trial period.
Experimental design
The study was conducted according to a completely randomized design and took place on a farm in Selçuklu (Konya, Türkiye) at coordinates 38°1′36″N, 32°30′45″E. On arrival, one hundred-twenty-five female Japanese quails (Coturnix coturnix Japonica), were weighed (244 ± 12.3 g) and randomly allocated into five treatment groups with five replicates. All pens were standardized to identical size (30 × 45 cm) and upheld in well-ventilated, hygienic, and sanitized conditions. The ambient room temperature was controlled at 22°C (± 2.0), with a 16-h lighting schedule programme imposed. Additionally, each enclosure was outfitted with separate feeders and drinkers, allowing unrestricted access to both food and water.
Throughout the 12-week duration, all quails were provided with the identical basal diet comprising corn and soybean meal, characterized by a crude protein content of 200 g/kg, metabolizable energy of 12.14 MJ/kg, and copper content of 7.20 mg/kg, as detailed in Table 1. The basal diet containing 7.20 mg/kg of copper was employed for the diet devoid of supplemental hydroxychloride copper (54% Cu). According to the literature reviewed, the hydroxychloride Cu was incorporated into the basal diet at the expense of corn to meet the desired concentration of Cu for the corresponding treatment at inclusion rates of 15, 30, 45 and 60 mg/kg Cu. The mash-formulated basal diet was formulated with the nutrient requirements recommended for laying quails according to the National Research Council (1994), except for Cu. The chemical composition of the basal diet was analysed using the methods specified by AOAC (2006). The basal diet's chemical composition was determined by subjecting samples to incineration and drying to ascertain ash content (method 942.05 analysing protein and fat levels using Kjeldahl (method 990.03) and Soxhlet techniques (method 2003.06), and analysing moisture content (method 2001.12) through drying at 105°C. Table 1 outlines the chemical composition and ingredients of the basal diet.
Table 1. Basal diet and its nutrient content (as fed)
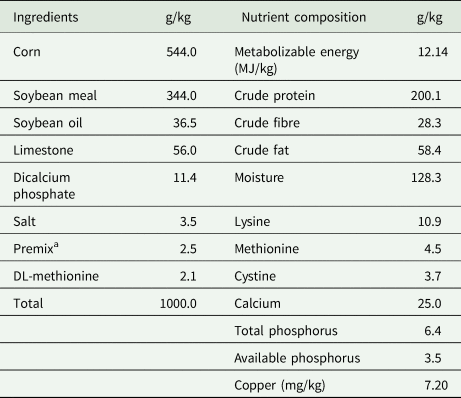
a Premix (vitamin-mineral mixture) as contained per kg feed: vitamin A, 20000 IU; vitamin D3, 10000 IU; vitamin E, 125 mg; vitamin K3, 5 mg; vitamin B12, 0.0275 mg; biotin, 0.30 mg; folic acid, 2.5 mg; nicotinic acid, 112.5 mg; pantothenic acid, 37.5 mg; pyridoxine, 3.75 mg; riboflavin, 10 mg; thiamin, 5 mg; iodine, 3 mg; iron, 50 mg; manganese, 60 mg; zinc, 50 mg; selenium, 0,75 mg.
Evaluation of performance indicators and egg production
To establish performance parameters, all experimental quails (n = 125) underwent individual weighing at the beginning and conclusion of the study (± 0.01 g). Subsequently, changes in body weight (g) were determined based on the weight difference. Feed intake (g/quail/day) was calculated following the methodology outlined by Olgun et al. (Reference Olgun, Gül, Kılınç, Yıldız, Çolak and Sarmiento-García2022) which involved monitoring the total feed dispensed and the remaining amount in the feed boxes. Egg production was evaluated by dividing the daily egg count by the total laying quails and multiplying the result by 100, expressing the result as per egg/100 quails. Additionally, every egg collected during the final three days of the study underwent weighing using a high-precision balance (±0.01 g) to ascertain egg weight. Egg mass (g/quail/day) and feed conversion ratio were determined according to the procedures delineated by Sarmiento-García et al. (Reference Sarmiento-Garcia, Olgun, Kılınç, Sevim and Gökmen2023).
Assessment of eggshell quality parameters
A total of 300 eggs were collected and sent to the Egg Quality Laboratory at the Faculty of Agriculture, Selcuk University, Konya, Turkey, for evaluation of eggshell quality. Eggs gathered during the final three days of the trial were assessed for both internal and external quality parameters under ambient temperature conditions. Throughout the experiment, any instances of broken, damaged, or cracked eggs were noted and expressed as a percentage of the total egg count (n = 300). Shell strength was measured using the Egg Force Reader (Orka Food Technology Ltd., Ramat Hasharon, Israel) applied to the blunt part of the egg. Eggshell thickness was determined utilizing a micrometre (Mitutoyo, 0.01 mm, Japanese), with measurements taken at three different locations on the shell (equator, blunt, and pointed), and subsequently averaged. Relative eggshell weight was computed by weighing the cleaned and dried shells and dividing them by their respective egg weights.
Determination of yolk MDA and DPPH values
Malondialdehyde (MDA) and 1-diphenyl-2-picrylhydrazyl (DPPH) concentrations were determined on 100 fresh eggs to establish the lipid peroxidation of the yolk. The thiobarbituric acid reactive substances (TBARS) test was carried out to establish MDA value using a modified method reported by Kilic and Richards (Reference Kilic and Richards2003) and Sarmiento-García et al. (Reference Sarmiento-García, Palacios, González-Martín and Revilla2021), where each sample was measured in triplicate. MDA concentration was quantified by measuring absorbance at 530 nm wavelength using a spectrophotometer (Perkin Elmer, USA), based on a blank curve of MDA containing 1 ml of trichloroacetic acid (TCA) solution (7.5% TCA, 0.1% EDTA, 0.1% Propyl gallate) and 1 ml of thiobarbituric acid (TBA) solution (0.02 M). The results were reported as μmol MDA/kg yolk.
The antioxidant capacity of the hydrolysates obtained was checked using a modified technique described by Sacchetti et al. (Reference Sacchetti, Maietti, Muzzoli, Scaglianti, Manfredini, Radice and Bruni2005) and Olgun et al. (Reference Olgun, Gül, Kılınç, Yıldız, Çolak and Sarmiento-García2022), which relies on the scavenging effect of DPPH radicals. Each sample was measured in triplicate to establish the average value. Absorbance at 517 nm was measured using a spectrophotometer (Perkin Elmer precisely UV/VIS Spectrometer) and compared against a blank curve substituted with 95% ethanol. Equation (1) was utilized to assess the scavenging effect:

Assessment of bone biomechanical properties
As the experiment ended, one female quail from each replicate (n = 25) was randomly euthanized via cervical dislocation. The right tibia was then removed, cleaned of tissue and cartilage, and stored at −20°C until the determination of bone biomechanical properties and mineral concentration. Before the examination, the samples were allowed to reach room temperature over 6 h in a controlled air environment. Cortical bone thickness, cortical bone cross-sectional area, shear force, and shear stress of bone were assessed according to protocols outlined by Gül et al. (Reference Gül, Olgun, Yıldız, Tüzün and Sarmiento-García2022), Armstrong et al. (Reference Armstrong, Flowers, Spears and Nielsent2002), and Wilson and Ruszler (Reference Wilson and Ruszler1996).
Analysis of mineral content in faeces and tibia
The mineral levels in faecal (n = 25) and tibia tissue (n = 25) samples were analysed using a wet digestion method following the protocol by Olgun and Yıldız (Reference Olgun and Yıldız2014). Samples from faeces and tibia, previously dried at 105°C for 24 h, were weighed (0.3 ± 0.01 g) and placed on Teflon-coated digestion plates (Milestone, Sorisole, Bergamo, Italy). A mixture of nitric acid (5 ml, 63.01 M) and perchloric acid (3 ml, 70%) was added to each plate. The samples were then heated in a microwave oven at 190°C for 40 min (CEM Corp, 3100 Smith Farm Road, Matthews, NC, USA). After cooling, the resulting supernatant solution was diluted with distilled water to 50 ml. Subsequently, 0.1 g of each ash sample was weighed to determine the mineral content, including concentrations of copper, calcium, phosphorus, manganese and zinc, using inductively coupled plasma atomic emission spectrometry (ICP-OES) with a Thermo Scientific 7200 analyser (Thermofisher Scientific, Waltham, United States).
Statistical analysis
The data were analysed using analysis of variance (ANOVA) in SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Each cage replicate was considered as the experimental unit, and values presented in the Tables are displayed as means with Standard error means (S.E.M). Statistical significance was set at P < 0.05. Linear, quadratic, and cubic regressions were employed to examine the relationship between the dependent variable and increasing copper levels across all parameters.
Results
Performance indicators and egg production
Table 2 illustrates the effects of dietary Cu levels on the performance traits of laying quails. No statistically significant differences in performance traits were found among the experimental groups (P > 0.05). The range of values for these parameters was as follows: final body weight (265–277 g), body weight gain (22–32 g), egg production (90.4–92.1 per egg/100 quails), egg weight (12.6–13.3 g), egg mass (11.4–12.1 g/quail/day), feed intake (31.4–33.7 g/quail/day), and feed conversion ratio (2.70–2.85).
Table 2. Effect of dietary Cu levels on performance in laying quails (n = 125)

s.e.m., Standard error means; L, Linear; Q, Quadratic; C, Cubic.
Eggshell quality
The eggshell quality parameters are expressed as damaged egg, egg-breaking strength, shell ratio, and shell thickness, as shown in Table 3. The effect of Cu supplementation in the diet of laying quails appeared to have no impact on eggshell parameters, as all values were similar (P > 0.05). Those values ranged from 0.2 to 1.6 per egg/100 eggs for damaged eggs, 12.0 to 12.9 N for egg-breaking strength, 8.18 to 8.72 g shell/ 100 g egg for shell ratio and 222 to 230 μm for egg-shell thickness.
Table 3. Effect of dietary Cu levels on eggshell quality (n = 300) in laying quails
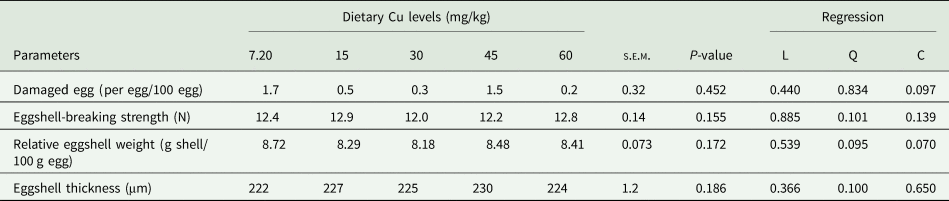
s.e.m., Standard error means; L, Linear; Q, Quadratic; C, Cubic.
Yolk DPPH and MDA values
The oxidative stability of the yolk measured as MDA and DPPH value is shown in Table 4. The MDA value was not impaired (P > 0.05) by Cu supplementation. Contrarily, a significant effect was described for the DPPH value, which significantly increased (P < 0.01) at 60 mg/kg of Cu compared to the rest of the experimental groups.
Table 4. Effect of dietary Cu levels on DPPH and MDA values of yolk (n = 100) in laying quails

s.e.m., Standard error of means; L, Linear; Q, Quadratic; C, Cubic; DPPH, 2,2-diphenyl-1-picrylhydrazyl; TBARS, thiobarbituric acid reactive substances.
Tibia biomechanical traits
Data on tibia biomechanical properties are shown in Table 5. Cortical bone thickness (0.323−0.378 mm), cortical bone cross-sectional area (1.22–1.30 mm2), shear force (131–154 N), and shear stress (100–119 N/mm2) were not affected by the Cu level (P > 0.05). Nevertheless, a linear regression (P < 0.05) was set for cortical bone thickness, which was linearly reduced from 0.378 to 0.323 mm, with increasing Cu content in the diet.
Table 5. Effect of dietary Cu levels on biomechanical traits of tibia (n = 25) in laying quails
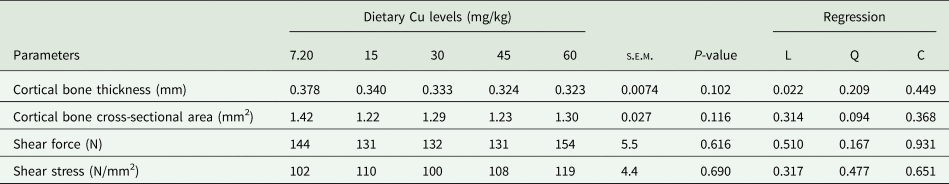
s.e.m., Standard error means; L, Linear; Q, Quadratic; C, Cubic.
Tibia mineralization
Table 6 summarizes the tibia mineral concentration of laying quails in response to rising levels of Cu supplementation. Tibia Cu content decreased linearly (P < 0.01) with increasing Cu levels in the diet, resulting in higher values in the non-supplemented quails than in the quails receiving 30, 45 and 60 mg/kg of Cu. Contrarily, as the dietary Cu level increased (P < 0.01) the tibia manganese content linearly rose, recording the highest value at 60 mg/kg Cu than those reported for 15 mg/kg Cu diet and non-supplemented diets. Similarly, zinc concentration increased with enhanced Cu supplementation levels, and this increase was significant (P < 0.01) at 60 mg/kg Cu compared to other levels. The effect of Cu supplementation on tibia calcium content appeared to be not significant (P > 0.05). There was a significant effect (P < 0.01) of Cu supplementation on tibia phosphorus content, quails receiving 15 and 30 mg/kg diets had considerably higher P levels than the quails receiving 45 and 60 mg/kg Cu.
Table 6. Effect of dietary Cu levels on mineral contents of tibia (n = 25) in laying quails
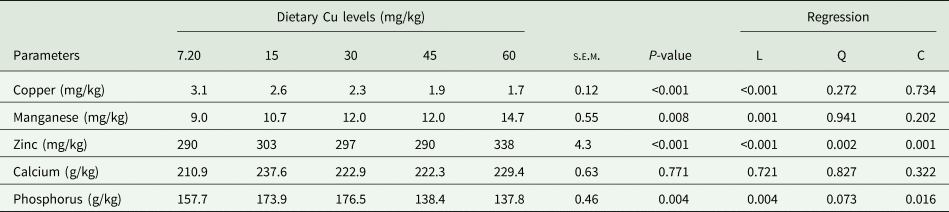
s.e.m., Standard error means; L, Linear; Q, Quadratic; C, Cubic.
Mineral excretion
Faecal mineral content (Cu, manganese, zinc, calcium and phosphorus concentration) in response to the Cu diets are presented in Table 7. Increasing the dietary Cu level from 7.20 to 60 mg/kg resulted in a linear increase in faecal Cu level from 18 to 56 mg/kg. Contrarily, the study indicated that the groups supplemented with Cu had considerably lower (P < 0.01) zinc and manganese (280–281 mg/kg) faecal content than the non-supplemented group. Faecal calcium content was not affected by dietary Cu level (P > 0.05), whereas a quadratic regression (P < 0.05) was observed with minimum values at 15 mg/kg of Cu. Faecal phosphorus was found to be significantly higher in the non-supplemented group compared to the laying quails supplemented with 15 and 30 mg/kg Cu.
Table 7. Effect of dietary Cu levels on mineral contents of faecal (n = 25) in laying quails
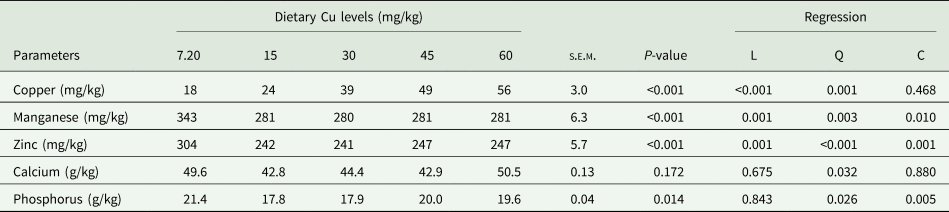
s.e.m., Standard error means; L, Linear; Q, Quadratic; C, Cubic.
Discussion
Trace elements, including Cu, are crucial in the diet of poultry to ensure optimum performance, since these elements due to these elements are involved in several biochemical processes (Olukosi et al., Reference Olukosi, Van Kuijk and Han2019). However, the optimal dose of Cu supplementation to ensure optimal performance without compromising the environment is still controversial (Cufadar et al., Reference Cufadar, Curabay, Gökmen, Bahtiyarca and Sevim2022). In this study, the addition of hydroxychloride copper did not yield any discernible impact on performance parameters. Comparable results have been reported by previous researchers in studies involving laying quails (Kara et al., Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021; Cufadar et al., Reference Cufadar, Curabay, Gökmen, Bahtiyarca and Sevim2022) and broilers (Nguyen et al., Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020). On egg production in laying hens, similar to the current findings, Olukosi et al. (Reference Olukosi, Van Kuijk and Han2019) observed no effect of Cu hydroxychloride supplementation, which is in line with that described by Cufadar et al. (Reference Cufadar, Curabay, Gökmen, Bahtiyarca and Sevim2022) and Kara et al. (Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021) when organic Cu was added to the diet of quails. Olukosi et al. (Reference Olukosi, Van Kuijk and Han2019) proposed that the absence of differences could be attributed to the hens being at peak production, a circumstance consistent with the age of the quails in this study. In this research, the concentration of Cu in the basal diets approached the minimal requirement (6 mg/kg) reported for quails by the National Research Council (1994). Therefore, the comparative performance and egg production traits of quails fed a diet without copper supplementation imply that the basal diet, primarily composed of corn, did not exhibit a significant Cu deficiency. Therefore, based on these results and as pointed out by previous authors (Nguyen et al., Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020; Cufadar et al., Reference Cufadar, Curabay, Gökmen, Bahtiyarca and Sevim2022), it could be suggested that additional dietary Cu supplementation is not necessary to ensure optimal growth or egg production in laying quails.
Egg-shell quality remains a critical problem for avian production which accounts for large economic losses for the avian industry. Therefore, it is important to ensure that shell quality parameters are preserved (Gül et al., Reference Gül, Olgun, Yıldız, Tüzün and Sarmiento-García2022). The current research found that the hydroxychloride Cu supplementation did not affect the eggshell quality (in terms of damaged egg, eggshell breaking strength, relative eggshell weight, and eggshell thickness). Similarly, Olukosi et al. (Reference Olukosi, Van Kuijk and Han2019) described an absence of differences in eggshell thickness and relative eggshell weight of the eggs from hydroxychloride Cu-fed hens, which follows those described by El Sherif et al. (Reference El Sherif, Fouad, Nassar, Wahba, Elsabagh and El-Iraqi2019). These findings align with those reported by Kara et al. (Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021) who stated that the addition of propionate Cu (organic form) at the level of 80 mg/kg did not affect eggshell quality. However, Olgun et al. (Reference Olgun, Yıldız and Şentürk2020) reported that administration of 20 mg/kg organic Cu to the basal diet (35.95 mg/kg Cu) decreased eggshell breaking strength and shell index, but conversely increased eggshell thickness. Similarly, Cufadar et al. (Reference Cufadar, Curabay, Gökmen, Bahtiyarca and Sevim2022) demonstrated that incorporating high levels of organic copper (150 or 225 mg/kg) into the diets of laying quails enhanced eggshell thickness and eggshell ratio without affecting eggshell breaking strength. As can be perceived, there are several discrepancies between previous studies. Factors such as laying age, basal Cu doses, or the supplementation with organic, inorganic, or chelated Cu sources, among others, can impact the perceived effects. Nonetheless, the consensus is that using organic or hydroxychloride Cu sources rather than inorganic sources could result in improved eggshell quality, which may or may not, be projected in eggshell thickness (Olukosi et al., Reference Olukosi, Van Kuijk and Han2019). In any case, the lack of differences in eggshell traits suggests that the Cu provided in the basal diet would be enough to ensure the eggshell quality of the laying quails.
Cu serves as a vital component of the antioxidant system, enhancing the activity of antioxidant enzymes (Kara et al., Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021), but, excess of dietary Cu could act as a prooxidant due to the formation of hydroxyl radicals leading to lipid peroxidation (Ali, Reference Ali2018; Lin et al., Reference Lin, Guo, Liu, Wang, Su, Yu and He2020; Nguyen et al., Reference Nguyen, Kheravii, Wu, Roberts, Swick and Toghyani2022). In the present investigation, the addition of Cu hydroxychloride at the maximum level (60 mg/kg) resulted in an improvement of the DPPH value in comparison to the rest of the experimental diets, without promoting oxidative stress in any case. Those results are partially following those reported by Nguyen et al. (Reference Nguyen, Kheravii, Wu, Roberts, Swick and Toghyani2022). These results suggest that Cu hydroxychloride, even at high doses, would have a reduced release of Cu ions, resulting in a significantly reduced reactivity potential and less production of reactive oxygen species leading to less oxidation (Lin et al., Reference Lin, Guo, Liu, Wang, Su, Yu and He2020). Scarce data about the effect of Cu hydroxychloride dietary supplementation on the antioxidant capacity of the yolk have been reported. Most previous studies have focused on the effect of other dietary sources of Cu (inorganic or organic) or the combination of hydroxychlorinated minerals on the antioxidant capacity of different tissues. For example, Kara et al. (Reference Kara, Kocaoğlu Güçlü, Şentürk, Eren and Baytok2021) reported that the administration of Cu proteinate at the level of 80 mg/kg reduced yolk MDA. Abdullah et al. (Reference Abdullah, Masood, Zaneb, Rabban, Akba and Kuthu2022) noted an increase in serum SOD activity and a decrease in MDA values in broilers that had received Cu nanoparticles in the diet. Similar outcomes have been described by Wu et al. (Reference Wu, Dai, Hua, Hu, Wang and Wen2019), who found increased antioxidant protection (measured by SOD and GSH-x activities) in the serum of broiler chickens supplemented with organic Cu, which agrees with those described by Ali (Reference Ali2018). These authors found a reduction of serum MDA value in quails fed with dietary-containing Cu (cupric sulphate pentahydrate) regardless of the doses. It would appear that, overall, dietary Cu would have some antioxidant effect on different tissues. On the other hand, El Sherif et al. (Reference El Sherif, Fouad, Nassar, Wahba, Elsabagh and El-Iraqi2019) proposed that the total antioxidant capacity in the serum of laying hens was enhanced as the dietary Cu sulphate was reduced, while Nguyen et al. (Reference Nguyen, Kheravii, Wu, Roberts, Swick and Toghyani2022) revealed that including 200 mg Cu/kg to the broiler diet did not affect liver MDA values. Nonetheless, it is important to note that the animals in the El Sherif et al. (Reference El Sherif, Fouad, Nassar, Wahba, Elsabagh and El-Iraqi2019) experiment were reared under stressful environmental conditions. The differences among studies are probably due to the dosage, the source of Cu involved, the age of the animals, and the environmental conditions.
The absorption of trace elements is crucial for the development of the skeletal system, potentially affecting overall bone health. In this sense, Cu is an important mineral to preserve the skeletal system as it serves as a cofactor for lysyl oxidase. This enzyme binds collagen and elastin proteins and is necessary to ensure structural integrity (Nguyen et al., Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020). Furthermore, the antioxidant capacity of Cu has a major role in neutralizing the radicals which stimulate the osteoclasts. Hence, a deterioration of the biomechanical parameters of the tibia suggests a deficit in Cu requirements. As previously stated, no alteration of tibial biomechanical traits linked to dietary Cu level was described, which agrees with the results of previous reports (Nguyen et al., Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020; dos Santos et al., Reference dos Santos, Augusto, Han, Sartori, Batistioli, Contin Neto, Ferreira Netto, Zanetti, Pasquali, Muro, Araujo, Basso, Guimarães, Takahira, Kim and Sartori2023). This observation supports the hypothesis that the Cu content of the basal diet would be enough to satisfy the requirements of laying quails.
Tissue mineral contents are commonly determined to assess mineral availability in livestock (Lin et al., Reference Lin, Guo, Liu, Wang, Su, Yu and He2020) and are a good marker of bone mineralization (Nguyen et al., Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020). Interestingly, a reduction in the Cu content of the tibia was reported as the dietary Cu level was increased. This fact could be related to its role in phytase inhibition, vitamin degradation, and increased oxidation which could indirectly result in reduced bioavailability and decreased absorption of Cu in the tibia, as reported by Lin et al. (Reference Lin, Guo, Liu, Wang, Su, Yu and He2020). The increase of Cu levels in faeces confirms these results. An increase in tibial zinc and manganese contents was only recorded in quails that had received the highest dietary Cu levels. At the same time, phosphorus showed the lowest values when dietary Cu levels were increased (45 and 60 mg/kg) which is by those described by Nguyen et al. (Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020). It has been described that high doses of Cu could impair the bioavailability of phosphorus by the formation of an insoluble phytate-C complex as occurs in this research. Moreover, dietary Cu did not alter the deposition of calcium which is following those reported by Nguyen et al. (Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020). However, according to the previous literature, the effects of dietary Cu on the tibia mineral content are controversial. Dos Santos et al. (Reference dos Santos, Augusto, Han, Sartori, Batistioli, Contin Neto, Ferreira Netto, Zanetti, Pasquali, Muro, Araujo, Basso, Guimarães, Takahira, Kim and Sartori2023), described that dietary Cu in hydroxychloride form (at a level of 150 mg/kg) did not affect the Cu and phosphorus contents of the tibia in broilers, but reduced the calcium and zinc, while Nguyen et al. (Reference Nguyen, Morgan, Roberts, Swick and Toghyani2020) found that the majority of tibia minerals assessed remained unaffected by Cu dietary supplementation. Lin et al. (Reference Lin, Guo, Liu, Wang, Su, Yu and He2020) suggested that the variations observed in previous research could stem from differences in factors such as the ages of the animals, forms and levels of copper supplementation, duration of the experiments, as well as the composition of the diets, among other factors. Nevertheless, our findings suggest that non-supplemented Cu diets are enough to ensure normal bone mineralization and absorption processes.
In the current study, dietary supplementation with Cu resulted in increased faecal excretion of Cu but decreased excretion of manganese, zinc, and phosphorus. As far as we know, there have been no studies investigating the effect of dietary copper on faecal mineral excretion in laying quails or hensSomewhat congruent with these observations, Nguyen et al. (Reference Nguyen, Kheravii, Wu, Roberts, Swick and Toghyani2022) observed that adding hydroxichloride Cu at high doses (200 mg/kg) to the broiler diet́ resulted in an increased Cu in distal ileum content, but did not influence the excretion of other minerals (calcium, phosphorus, zinc, and manganese). Mineral absorption is a complex process, and microminerals can use the same transport medium to be assimilated by the cell (dos Santos et al., Reference dos Santos, Augusto, Han, Sartori, Batistioli, Contin Neto, Ferreira Netto, Zanetti, Pasquali, Muro, Araujo, Basso, Guimarães, Takahira, Kim and Sartori2023). Consequently, even if the mineral content is equal, variations in the dietary components or the presence of chelating agents may lead to different results in the absorption process (Lin et al., Reference Lin, Guo, Liu, Wang, Su, Yu and He2020). Hence, additional study is warranted to validate the mechanism underlying mineral excretion.
Conclusions
Copper plays a crucial role in maintaining, developing, and promoting the growth and overall health of animals. However, there seems to be no consensus on the appropriate dose to improve the development of laying quails without compromising the environment. This study revealed that while dietary intake of hydroxychloride copper at a level of 60 mg/kg had a beneficial impact on egg yolk antioxidant status, the basal diet composed of corn and containing 7.20 mg/kg copper could be sufficient to meet the requirements for animal development, egg production traits, egg quality, and bone biomechanical properties of laying quails, without compromising any of these parameters. It should be noted that reducing the dietary inclusion of Cu to basal levels would result in less Cu excretion to the environment, which would contribute to its protection.
Acknowledgements
We acknowledge the contributions of Dr Nicholas Collins (School of Agriculture Food and Wine, The University of Adelaide, Adelaide, SA, Australia) who supervised these projects, and thank all the technical staff involved in the field experiments.
Author contributions
Esra T. Gül, Osman Olgun, Alpönder Yıldız, Behlül Sevim and Ainhoa Sarmiento-García contributed to the conceptualization, investigation, methodology, writing – original draft and writing – review and editing. Gözde Kılınç contributed antioxidant analysis. Veli Uygun and Fatih Gökmen contributed mineral analysis. Esra T. Gül, Osman Olgun and Ainhoa Sarmiento-García contributed to supervision, review and editing. All authors have read and approved the final version of the manuscript.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
None.
Ethical standards
The authors confirm the ethical policies of the journal, as noted on the journal's author guidelines page. The European National Research Council's guidelines for the Care and Use of Laboratory Animals were followed.










