Introduction
Wild edible plants have become increasingly popular with an increasing amount of research and species diversity in several parts of the world. Some of the reasons for their popularity are to improve the conservation and use of agricultural biodiversity (Tan et al., Reference Tan, Adanacioglu, Karabak, Aykas, Tas and Taylan2017), potential use in breeding programmes (Kapoor et al., Reference Kapoor, Mawal, Sharma and Gupta2020), several uses in local and traditional cuisine (Pieroni et al., Reference Pieroni, Nebel, Santoro and Heinrich2005) and particularly, their important beneficial health effects, which are mostly associated with their high content of micronutrients and phytochemicals.
The genus Asparagus includes many species belonging to the family Asparagaceae, which are distributed worldwide (Kanno and Yokoyama, Reference Kanno, Yokoyama and Kole2011). The genus consists of many diverse species with different plant forms (herbaceous perennials, woody shrubs, vines, photosynthetic stems, black or red berries) and reproductive behaviours (monoecious, dioecious, hermaphroditic), some of which are used for their ornamental value and foliage (i.e. A. plumosus Baker, A. densiflorus Kunth, A. virgatus Baker), while others are used for their medicinal properties (i.e. A. racemosus Willd., A. verticillatus L., A. adscendens Kunth). Among these species, A. officinalis L. is the only asparagus species cultivated on a global scale, and the rest grow spontaneously in climates suitable for their biological cycles (Boubetra et al., Reference Boubetra, Amirouche and Amirouche2017; Altunel, Reference Altunel2021).
Asparagus officinalis, a perennial crop, is considered to be one of the nutritionally well-balanced vegetables. It is a good source of essential minerals, vitamins, amino acids and dietary fibre (Lopez et al., Reference López, Rosa, Rincón, Ortuño, Periagoa and Martinez1996). Additionally, A. officinalis contains flavonoids (mainly rutin) and other phenolic compounds with strong antioxidant properties (Makris and Rossiter, Reference Makris and Rossiter2001). With the increasing demand for foods that are rich in composition, asparagus is now the focus of attention. Many studies have highlighted the attributes of spear quality and health-related compounds in asparagus, which may change in relation to genetics (Han et al., Reference Han, Fan, Cheng and Fu2008), environmental conditions (Maeda et al., Reference Maeda, Honda, Sonoda, Motoki, Inoue, Suzuki, Oosawa and Suzuki2010), agronomic factors (Siomos Reference Siomos2018), spear traits (section, length, thickness) (Slatnar et al., Reference Slatnar, Mikulic-Petkovsek, Stampar, Veberic and Horvat2018) and processing (Nindo et al., Reference Nindo, Sun, Wang, Tang and Powers2003).
A. acutifolius, a native plant species commonly found throughout the Mediterranean basin, has tender spears used as a vegetable (Anido and Cointry, Reference Anido, Cointry, Prohens and Nuez2008). A. acutifolius is present in vast areas of western Turkey (Davis, Reference Davis1984). It is also known for its fine flavour, commonly gathered from uncultivated areas, marketed at high prices in local markets and used in various traditional cuisines (Kaska et al., Reference Kaska, Deniz and Mammadov2018). This wild species is becoming an interesting niche crop for marginal rural areas in Europe and studies have been conducted to develop suitable cultivation techniques (Benincasa et al., Reference Benincasa, Tei and Rosati2007). In addition to its considerable capacity for adaptation, drought and cold resistance, it has recently been used in breeding programmes to cross it with A. officinalis to obtain cultivars resistant to Puccinia asparagi and Stemphylium vesicarium, which are common pathogens of A. officinalis (Kubota et al., Reference Kubota, Konno and Kanno2012; Castro et al., Reference Castro, Gil, Cabrera and Moreno2013; Mousavizadeh et al., Reference Mousavizadeh, Gil, Castro, Hassandokht and Moreno2021).
Despite this, many studies have examined the changes in spear quality attributes and health-related compounds in A. officinalis. However, there is little agricultural quality data available for A. acutifolius, with comparative studies between wild and cultivated species. Palmieri et al. (Reference Palmieri, Villari, Caruso, Orlando and Parente2008) conducted a comparative study between the wild type (two ecotypes of A. acutifolius) and one commercial cultivar of A. officinalis under the same growing conditions. The reported results showed that the spears of the Raviscanina and Marzano Appio varieties of A. acutifolius had a better chemical composition than those of A. officinalis. Ferrara et al. (Reference Ferrara, Dosi, Di Maro, Guida, Cefarelli, Pacifico, Mastellone, Fiorentino, Rosati and Parente2011) compared fresh spears of A. acutifolius (collected around southern Italy) with frozen spears of cultivated asparagus ‘UC800’ (obtained from the market) in terms of nutritional value, metabolic profile and radical-scavenging capacity. However, Gebczynski (Reference Gębczyński2007) suggested that the antioxidative compounds of green asparagus change depending on the processing before freezing and the storage period and conditions. Similarly, Palfi et al. (Reference Palfi, Jurković, Ćosić, Tomić-Obrdalj, Jurković, Knežević and Vrandečić2017) compared the total polyphenol content and antioxidant activity (AA) of wild (collected from four different locations) and cultivated asparagus (collected from four different locations, cultivar name was not detected). Sergio et al. (Reference Sergio, Boari, Di Venere, Gonnella, Cantore and Renna2021) compared the physical and chemical traits of wild (green and violet) and cultivated asparagus (green, violet and white; cultivar names detected) spears before and after steaming. They stated that for their plant material, samples of wild asparagus were collected from sunny and shady areas at one location, while their samples of green and violet cultivated asparagus were collected from another location, but white-cultivated asparagus was obtained from the market. The results indicated that wild asparagus had a higher phenolic compound composition, but lower values of chlorophylls, carotenoids and sugars (glucose, fructose and sucrose) than the cultivated ones.
To date, comparative literature reports regarding fresh spear quality traits and health-related compounds of wild and cultivated asparagus exist, while existing studies refer to samples obtained from markets, different locations and different harvest times. Additionally, there are no data available on seasonal variations in these comparative studies. Considering all the above remarks, the specific aims of this study were: (i) to compare fresh spear quality traits and health-related compounds in wild and cultivated asparagus for two consecutive years under the same climate conditions, (ii) to evaluate the importance of seasonal variations in spear quality traits and health-related compounds of wild and cultivated asparagus, by considering the same three harvest periods. The more general goal was to increase the knowledge about wild asparagus spear quality with seasonal changes for breeding purposes.
Materials and methods
Plant material and cultural practices
For the cultivated asparagus, A. officinalis cv. ‘Atlas F1’ (Walker Brothers Inc. Seed Company, USA) was used as plant material. Field trials were conducted for two consecutive years (2018 and 2019) in the experimental fields of the Odemis Vocational Training School at Ege University, Izmir, Turkey (latitude 38°12′N, longitude 27°52′E, altitude 111 m a.s.l.). The crowns of the cultivar ‘Atlas F1’ were 5 years old at the beginning of the experiment. The soil was a loamy sand with 1% organic matter, 0.03% total salt, 2.7% lime and pH of 7.15. The trials were conducted using a completely randomized block design with three replicates. Drip irrigation was applied as needed, and weeds were manually controlled mechanically. Fertilization and plant protection were performed according to standard methods.
Spears of wild asparagus (A. acutifolius L.) were collected from the same location as the cultivated asparagus (Table 1) for two consecutive years (2018 and 2019). Plant material was identified at Ege University, Faculty of Agriculture, Department of Horticulture, Izmir, Turkey.
Table 1. Sampling location and harvest dates of the cultivated and wild asparagus spears

In the first year of the experiment, spears emerged from the soil at the end of February, and the harvest started from early March to mid-April. In the second year of the experiment, the spears emerged from the soil in early March, and the harvest started from mid-March to early May when the spears were at least 20 cm above the soil surface. Cultivated and wild spears were sampled three times during the entire harvesting period: at the beginning, in the middle and at the end of the same period (Table 1). Straight, well-formed spears were harvested when the heads were tight with close bracts before they ‘ferned out’. The spears with broken heads that were damaged and curled were removed. Because the spear portion, thickness and length were related to the quality parameters, only spears with lengths of 20–25 cm and base diameters of 15–20 mm were sampled for analysis. After harvest, the spears were wrapped in plastic and immediately placed on ice for transport to the laboratory at Ege University, where analyses were performed. Base sections (approximately 4–5 cm) of spears were discarded. Wild and cultivated asparagus spears were cut into three segments for analysis: tip (0–6 cm, measured from the apex of the spear), middle portion (6–12 cm) and base (12–18 cm) following the method proposed by Flores-Rojas et al. (Reference Flores-Rojas, Sánchez, Pérez-Marín, Guerrero and Garrido-Varo2009). They were then washed first with tap water and twice with deionized water. Excess water was removed using a domestic salad spinner and the following experiments were conducted.
Quality attributes
The colour of the asparagus spears was determined using a colourimeter (CR-400; Minolta Co., Osaka, Japan), which provided CIE L* a* b* values. Chroma (C*), which indicated the intensity or colour saturation, and hue angle (h°), which was expressed as follows: 0° (red-purple), 90° (yellow), 180° (bluish-green) and 270° (blue) (McGuire, Reference McGuire1992) and were calculated using the following equations:
The total soluble solid (TSS) content of asparagus spear juice was determined using a digital refractometer (PR-1; Atago, Tokyo, Japan) and expressed as a percentage. matter (DM) content was determined by drying the samples in an oven (Memmert, Germany) at 65°C until a constant weight was obtained and calculated based on the percentage of weight loss (AOAC, 1990).
Chlorophyll a (CHL a) and chlorophyll b (CHL b) were extracted from fresh asparagus spears using acetone (85%) and homogenized using an Ultra-Turrax homogenizer for 3 min. The absorbance was measured using a UV/Vis spectrophotometer (Carry Bio 100, Varian, Australia) at 645 and 663 nm. CHL a, CHL b and total CHL content were calculated by Lichtenthaler and Wellburn (Reference Lichtenthaler and Wellburn1983) and expressed as mg/100 g on a fresh weight basis.
Total sugars and sugar fractions
Asparagus spear samples were mixed with ultrapure water (Millipore 18.2 ΩΏ), homogenized (Ika Ultra-Turrax T18 Basic, Germany) at an intermediate speed, filtered through filter paper and then transferred to falcon tubes and filtered into falcon tubes. Samples taken from the filtrate via syringe were passed through a 0.20 μm nylon filter and injected into vials. A 20 μl sample taken from vials was analysed using a Dionex UltiMate 3000 Series UHPLC (Thermo Scientific, USA) with a refractive index detector (RafrateMax521, Erc Inc., Japan) and a Hypersil GOLD Amino (150 × 4.6 mm) column at a flow rate of 0.1 ml/min (Chinnici et al., Reference Chinnici, Spinabelli, Riponi and Amati2005). The results were obtained by interpolating the data in graphs derived from glucose, fructose and sucrose standards, and were expressed as g/kg fresh weight.
Total phenolic content and antioxidant activity
Total phenolic content (TPC), AA and asparagus spear extracts were prepared according to the method of Thaipong et al. (Reference Thaipong, Boonprakob, Crosby, Cisneros-Zevallos and Byrne2006), with some modifications for TPC and AA (in methanol extract) analysis. Asparagus tissue (5 g) was mixed with 25 ml methanol and homogenized using the Ultra-Turrax homogenizer (Ultra-Turrax T18 Basic, Ika, Germany). The homogenates were kept at 4°C for 14–16 h and then centrifuged at 15.000 rpm for 20 min using a centrifuge. The TPC was determined using the Folin–Ciocalteu method, according to Zheng and Wang (Reference Zheng and Wang2001), with an incubation time of 120 min for colour development. The absorbance was measured at 725 nm using a spectrophotometer and the results were expressed as milligram gallic acid equivalent (GAE) per 100 g of fresh weight (FW) with reference to a gallic acid (0–0.1 mg/ml) standard curve.
The method described by Benzie and Strain (Reference Benzie and Strain1996) was used to ascertain at the ‘Ferric Reducing Antioxidant Power’ (FRAP) analysis. Using this method, the reductants (antioxidants) in the sample reduced the Fe (III)/tripyridyltriazine complex to its blue ferrous form. Absorbance of the reaction mixture was measured at 593 nm using a spectrophotometer. The final results are expressed in μmol Trolox equivalents (TE)/g FW, with reference to a Trolox (25–500 μmol/l) standard curve.
Statistical analysis
The experimental design was completely randomized for cultivated plants, with three replicates. For quality attributes, sugar composition and health-related assays, five spears were analysed for wild and cultivated plants, and all assays were performed in triplicates. The tip, middle and base spear portion data were then averaged. Statistical analysis of variance (ANOVA) was performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA). Data from 2018 and 2019 were analysed separately. A split-plot model with three replicates was used for variance analysis of all parameters, where season was attributed to the main plots and species to the sub plots. Significant differences among groups were determined using Duncan's multiple range test at P < 0.05.
Results
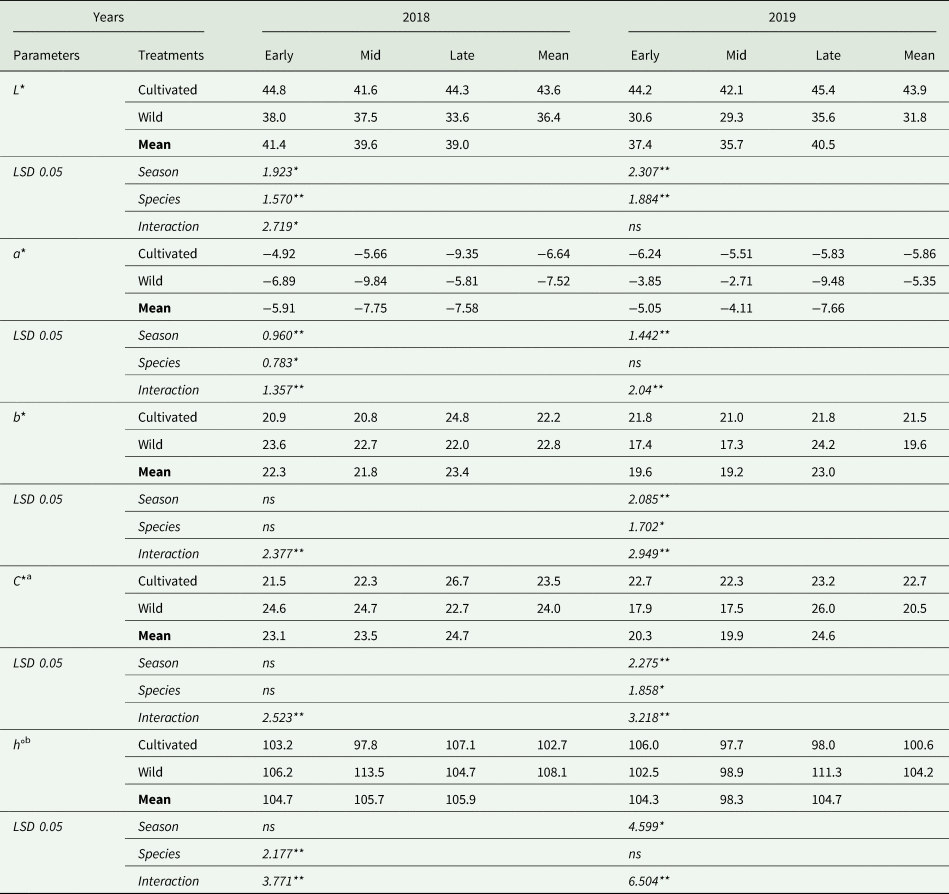
The colour characteristics of the cultivated and wild asparagus spears are presented in Table 2. In 2018, spear L* values were significantly affected by season, species (P < 0.05 and P < 0.01, respectively) and the interaction between season and species (P < 0.05). Early- and late-season harvests of cultivated asparagus had the highest L* values, whereas the late-season harvest of wild asparagus showed the lowest L* values. In the second year of the experiment, the statistical analysis revealed differences only between the main factors (P < 0.01). Cultivated asparagus increased the L* value by an average of 38% compared to wild asparagus. It can also been seen that during the harvest season, the L* value in spears declined from 37.4 in early-harvest season to 35.7 in mid-harvest season, and then increased again in late-harvest season (40.5).
Table 2. Colour characteristics of cultivated and wild fresh asparagus spears
ns, not significant. The significance and LSD values of season, species and interactions are in italic.
a Chroma.
b Hue angle.
* P < 0.05; **P < 0.01.
The effects of the main factors and their interactions on the a* value were statistically significant (P < 0.01, P < 0.05 and P < 0.01, respectively) in 2018. However, in 2019, species had no effect on the a* value, even though the effects of season and interaction were found to be significant (P < 0.01). The highest a* value was obtained from the mid-season harvest of wild asparagus (−9.84), followed by the late-season harvest of cultivated asparagus (−9.35) in 2018, although the late-season harvest of wild asparagus (−9.48) resulted in the highest a* value in 2019.
The b* colour parameter was significantly (P < 0.01) affected by the interaction of the main factors in the first year of the experiment; however, in 2019, both the main factors and their interactions were found to be significant (P < 0.01, P < 0.05 and P < 0.01, respectively). The b* colour parameter changed between 20.8 (mid-season harvest of cultivated asparagus) and 24.8 (late-season harvest of cultivated asparagus), in 2018; and between 17.3 (mid-season harvest of wild asparagus) and 24.2 (late-season harvest of wild asparagus), in 2019.
Season and species had no effect on the C* value of asparagus spears; however, the C* value was significantly (P < 0.01) affected by the interaction in 2018. The late-season harvest of cultivated asparagus (26.7), mid-season harvest of wild asparagus (24.7) and early season harvest of wild asparagus (24.6) had similar and higher C* values than the others. In the second year of the experiment, both the main effects and the interaction between the experimental factors on the C* value were found to be statistically significant (P < 0.01). Late-harvest of wild asparagus and late-harvest of cultivated asparagus gave the highest C* values, whereas early- and mid-season harvests of wild asparagus had the lowest values.
The effects of species and interaction on h° values were significant (P < 0.01) in 2018. The mid-season harvest of wild asparagus had the highest h° value, whereas the mid-season harvest of cultivated asparagus had the lowest. In the second year of the experiment, the species had no effect on the h° value, whereas the effects of season and interaction were found to be significant (P < 0.01). The highest h° value was obtained from the late-season harvest of wild asparagus and the lowest h° value was found in the mid-season harvest of cultivated asparagus.
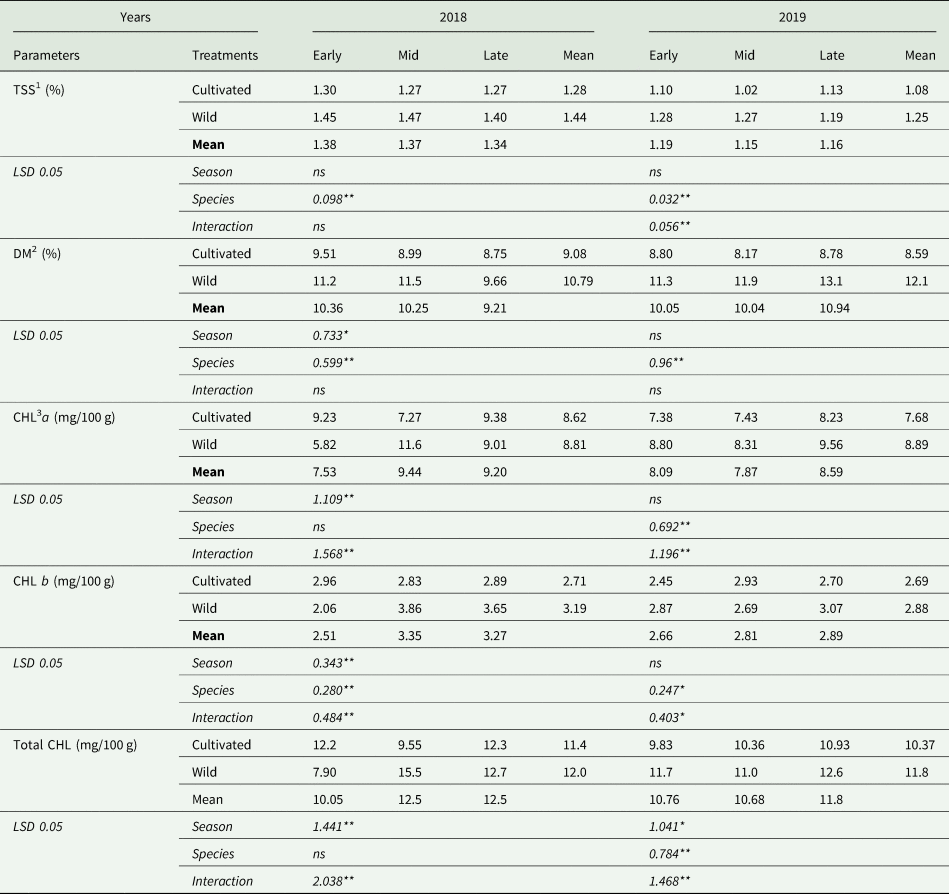
The TSS, DM and chlorophyll content of the experimental factors are presented in Table 3. In the first year of the experiment, the TSS content was significantly affected by species (P < 0.01). The TSS content in the spears of wild asparagus was higher TSS content with 1.44%, whereas cultivated asparagus spears had a lower TSS (1.28%). In the second year of the experiment, the effects of species and interaction on TSS content were significant (P < 0.01). Early- and mid-season harvests of wild asparagus had similar values and higher TSS contents (1.28 and 1.27%, respectively). The lowest TSS content was obtained from the mid-season harvest of wild asparagus spears (1.02%).
Table 3. Total soluble solid, dry matter and chlorophyll contents of cultivated and wild fresh asparagus spears
ns, not significant. The significance and LSD values of season, species and interactions are in italic.
1 Total soluble solid.
2 Dry matter content.
3 Chlorophyll.
* P < 0.05; **P < 0.01.
DM content was significantly affected by season (P < 0.05) and species (P < 0.01) in 2018; however, in 2019, statistical analysis revealed differences only between species (P < 0.01) (Table 3). According to the average results, in the first year of the experiment, the season declined in the following order: early season (10.35%) > mid-season (10.26%) > late-season (9.21%). It was also noted that wild asparagus spears had a higher DM content (19% in 2018 and 41% in 2019) than cultivated asparagus spears.
The CHL a content was significantly affected by the season and its interaction (P < 0.01) in the first year of the experiment. However, in 2019, the statistical analysis revealed differences between the species and the interaction (P < 0.01) (Table 3). The highest CHL a content was obtained from a mid-season harvest of wild asparagus, with 11.6 mg/100 g, while early-season harvest of cultivated asparagus (5.82 mg/100 g) had the lowest CHL a content. In the second year of the experiment, late-season and early-season harvests of wild asparagus had similar values and higher CHL contents than all others (9.56 mg/100 g and 8.80 mg/100 g, respectively). The lowest CHL a content was obtained in the early harvest season of the cultivated asparagus spears (7.38 mg/100 g).
The effects of the main factors and their interactions on CHL b content were found to be statistically significant (P < 0.01) in 2018; however, in 2019, the season had no effect on CHL b content, whereas the effects of species and the interaction were found to be significant (P < 0.05 and P < 0.01, respectively). The highest CHL b content was obtained from the mid-season harvest of wild asparagus (3.86 mg/100 g), followed by the late-season harvest of wild asparagus (3.65 mg/100 g) in 2018, even as the late-season harvest of wild asparagus (3.07 mg/100 g) gave the highest CHL b content in 2019.
Species showed no effect on the total CHL content of asparagus spears, but was significantly affected by season and interaction (P < 0.01) in 2018. The mid-season harvest of wild asparagus resulted in the highest CHL content compared to all others (15.5 mg/100 g). In the second year of the experiment, both the main effects and interactions between the experimental factors on the total CHL content were found to be statistically significant (P < 0.05, P < 0.01 and P < 0.01, respectively). Late (12.6 mg/100 g) and early harvests (11.7 mg/100 g) of wild asparagus gave the highest total CHL content.
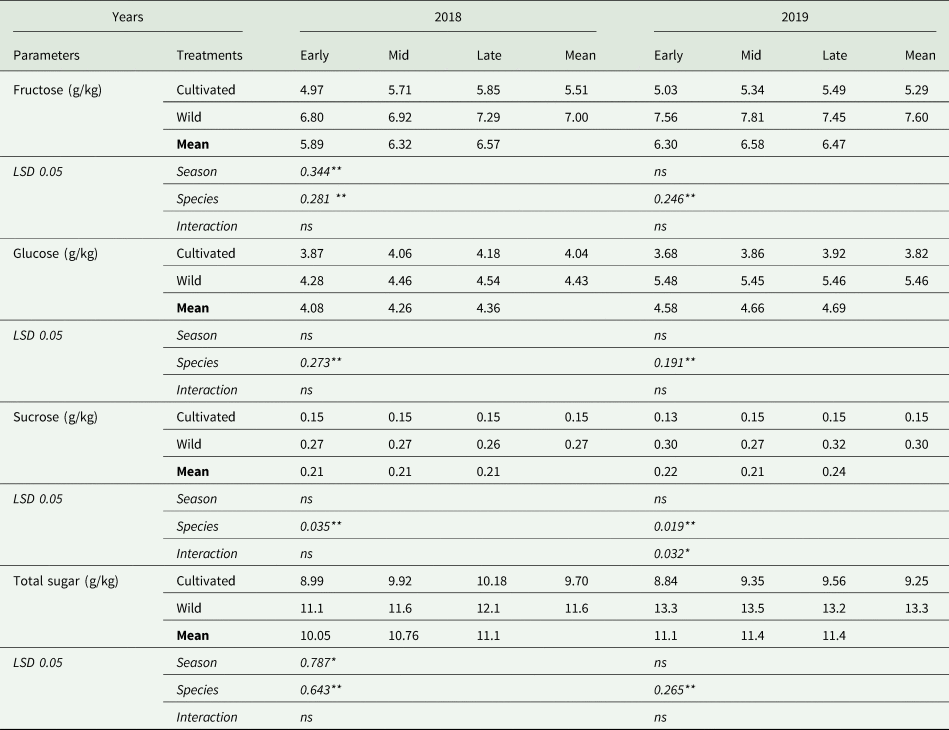
Table 4 presents the sugar fractions of cultivated and wild asparagus during the early, mid and late harvest seasons. The effects of season and species on fructose content were found to be significant in 2018, whereas they were only significantly affected by species in 2019 (P < 0.01). The lowest fructose content (5.89 g/kg) was obtained from the early season and it increased slightly towards to the late season (6.57 g/kg), in the first year of the experiment. It was also noted that the fructose content of wild asparagus spears was 27% (in 2018) and 44% (in 2019) higher than that in cultivated asparagus spears.
Table 4. Sugar fractions of cultivated and wild fresh asparagus spears
ns, not significant. The significance and LSD values of season, species and interactions are in italic.
* P < 0.05; **P < 0.01.
The effect of species on glucose content was significant (P < 0.01) in both years (Table 4). Similar to the fructose content, the wild asparagus spears had a higher glucose content of 10% in the first year of the experiment and by 43% in the second year of the experiment.
In the first year of the experiment, sucrose content was significantly affected (P < 0.01) by the species (Table 4). Wild asparagus spears had a sucrose content of 0.27 g/kg, which was higher by 80% than that in cultivated asparagus (0.15 g/kg). In 2019, the effects of species (P < 0.01) and the interaction between experimental factors on sucrose content were found to be statistically significant (P < 0.05). The lowest sucrose content was found in the early harvest season of cultivated asparagus (0.13 g/kg). The highest sucrose content was obtained from the late-season harvest of wild asparagus (0.32 g/kg), followed by early-season harvest of wild asparagus (0.30 g/kg).
The total sugar content was significantly affected by season (P < 0.05) and species (P < 0.01) in 2018; however, in 2019, the statistical analysis revealed differences only between species (P < 0.01) (Table 4). Wild asparagus increased the total sugar content in 2018 and 2019 by an average of 20 and 45%, respectively, compared to cultivated asparagus. In the first year of the experiment, it was also noted that the highest total sugar content was attained from the late-season harvest, with a mean content of 11.1 g/kg, and the total sugar content of asparagus spears diminished as follows: late-season harvest > mid-season harvest > early-season harvest.
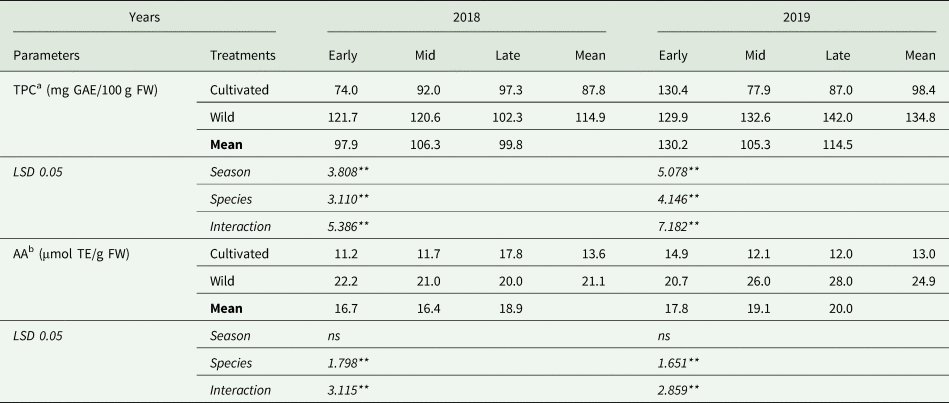
Table 5 presents TPC and AA of cultivated and wild asparagus. Both main effects and the interaction between the experimental factors on TPC were found to be statistically significant (P < 0.01) in 2018 and 2019. In the first year of the experiment, the highest TPC was obtained from early-season (121.7 mg GAE/100 g FW) and mid-season (120.6 mg GAE/100 g FW) harvests of wild asparagus and the lowest TPC was found in the early-season harvest of cultivated asparagus (74.0 mg GAE/100 g FW). In 2019, the highest TPC was attained from late-season harvest of wild asparagus (142.0 mg GAE/100 g FW), whereas, the mid-season harvest of cultivated asparagus with 77.9 mg GAE/100 g FW gave the lowest TPC. It was also noted that the TPC increased to a maximum in the mid-season harvest and then declined in the late-season harvest by up to 6.1% in 2018. The harvest season pattern of TPC was different in 2019. In mid-season, TPC in the asparagus spears fell to a lowest level (105.3 mg GAE/100 g FW) and then increased in the late season (114.5 mg GAE/100 g FW).
Table 5. Total phenolic contents and antioxidant activity of cultivated and wild fresh asparagus spears
ns, not significant. The significance and LSD values of season, species and interactions are in italic.
a Total phenolic content.
b Antioxidant activity.
** P < 0.01.
AA was significantly affected by species and the interaction between experimental factors (P < 0.01) in both years (Table 5). In 2018, early, mid and late-season harvests of wild asparagus had similar values and higher AA than all others (22.2, 21.0 and 20.0 μmol TE/g FW, respectively). The lowest AA was found in the early-season harvest of cultivated asparagus (11.2 μmol TE/g FW), followed by the mid-season harvest of cultivated asparagus (11.7 μmol TE/g FW). In the second year of the experiment, late (12.0 μmol TE/g FW) and mid-season (12.1 μmol TE/g FW) harvests of cultivated asparagus had the lowest AA, even as, late (28.0 μmol TE/g FW) and mid-season (26.0 μmol TE/g FW) harvests of wild asparagus gave the highest AA.
Discussion
The current study showed that L* values (significant for both years of the experiment), yellowness (+b*), C* (significant only in the second year of the experiment) and greenness (−a*) values (significant only in the first year of the experiment) of wild asparagus spears were significantly lower. h° values of wild asparagus spears, which were significant only in the first year of the experiment, were greater than those of cultivated asparagus spears. Seasonal variations had an unstable effect on colour traits, sometimes with significant differences, which showed some minor fluctuations over the course of the season in both years. According to the literature, the colour measurements of cultivated asparagus show variability among cultivars (Chen et al., Reference Chen, Ma, Dong, Song, Pu and Zhou2017) depending on the spear part, seasonal changes (Anastasiadi et al., Reference Anastasiadi, Collings, Shivembe, Qian and Terry2020) and light conditions (Wambrauw et al., Reference Wambrauw, Kashıwatan, Komura, Hasegawa, Narıta, Oku, Yamaguchi, Honda and Maeda2016). There are substances with different colours in asparagus, one of the most significant of which is chlorophyll, which is green. The coloured substances found in asparagus are directly and indirectly related to its taste, flavour, structure and nutritional value. Colour changes are expected to develop in cultivated and wild asparagus, and their genetic structure is important for the formation of colour pigments (Hulme, Reference Hulme1971; Goodwin, Reference Goodwin1976; Friend and Rhodes, Reference Friend and Rhodes1981; Wills et al., Reference Wills, McGlasson and Joyce1998). Climatic conditions such as temperature, the difference in day-night temperatures, light and so on, during seasonal changes, are considered to have an effect on the colours of asparagus. Climatic factors have been reported to affect many types of fruit and vegetables (Wills et al., Reference Wills, McGlasson and Joyce1998). To the best of our knowledge, this is the first comparative report on the colour characteristics of wild asparagus in relation to seasonal variations.
In the current study, wild asparagus had higher TSS and DM content than cultivated asparagus in both years. Similar results for DM content were reported by Sergio et al. (Reference Sergio, Boari, Di Venere, Gonnella, Cantore and Renna2021), who also observed higher amounts of DM content in wild asparagus spears than in cultivated ones. Moreover, Palmieri et al. (Reference Palmieri, Villari, Caruso, Orlando and Parente2008) pointed out that both TSS and DM contents of wild asparagus cultivars are slightly higher than those of the cultivated species. The lower DM content of cultivated asparagus could be due to the higher availability of nitrogen periodically applied through fertigation, whereas wild asparagus plants were not fertilized. These results are in agreement with the negative relationships observed in previous studies on available nitrate-nitrogen and DM in lettuce and other crops (Cardanes-Navarro et al., Reference Cardanes-Navarro, Adamowicz and Robin1999; Di Gioia et al., Reference Di Gioia, Gonnella, Buono, Ayala and Santamaria2017). The current study also showed that seasonal variations were found to have a limited effect on TSS and DM contents, where their contents remained stable, with no significant differences between the harvest periods, with the exception of DM content in the first year of the experiment, where a decline was seen from the beginning to the end of the harvest period. In contrast to our results, previous results with cultivated asparagus showed that the TSS content decreased from the beginning to the end of the harvest period, but it fluctuated markedly, depending on the weather conditions; for example, a combination of high temperature and rainfall shortage for a few days before the measurement resulted in a significant increase in TSS content (Zurawicz et al., Reference Zurawicz, Krzesinski and Knaflewski2008). Additionally, Shou et al. (Reference Shou, Lu and Huang2007) indicated that the DM content of cultivated asparagus showed minor fluctuations over the course of the season. To the best of our knowledge, this is the first report on the TSS and DM content of wild asparagus in relation to seasonal variation.
The current paper showed that higher CHL a (significant only in the second year of the experiment), CHL b (significant for both years of the experiment) and total CHL (significant only in the second year of the experiment) content were found in fresh wild asparagus spears. Although there were no significant differences between species in CHL a and total CHL content observed in the first year of the experiment, the values were slightly higher in wild asparagus than in cultivated asparagus. This result is in disagreement with Sergio et al. (Reference Sergio, Boari, Di Venere, Gonnella, Cantore and Renna2021), who compared wild and cultivated asparagus spears (before and after steaming) and demonstrated that higher contents of CHL a and CHL b were present in cultivated asparagus. Seasonal variations had an unstable effect on CHL content, sometimes with significant differences that showed minor fluctuations over the course of the season in both years. Shou et al. (Reference Shou, Lu and Huang2007) evaluated seasonal variations in the nutritional quality of cultivated asparagus and suggested that asparagus harvested in spring accumulates a higher level of CHL than that harvested in autumn. Similarly, Siomos (Reference Siomos2018) indicated that CHL levels increase with improved light conditions. Although the seasonal variation effects were significant, the absence of a stable decrease in chlorophyll values indicated that green colour was preserved throughout the season. Thus, the a* colour value is also the ‘−’ value during the season, indicating that the green colour tone is dominant. The current was the result of chlorophyll not being broken down during the growth period. To the best of our knowledge, this is the first comparative report on the CHL content of wild asparagus in relation to seasonal variations.
In the current research, higher fructose, glucose, sucrose and total sugar contents were found in fresh wild asparagus spears over two consecutive years. In contrast to the current research, Sergio et al. (Reference Sergio, Boari, Di Venere, Gonnella, Cantore and Renna2021) reported that wild asparagus showed lower values of sugars (glucose, fructose and sucrose) than cultivated asparagus. On the other hand, Palmieri et al. (Reference Palmieri, Villari, Caruso, Orlando and Parente2008) found that there was no clear difference between the two species (cultivated and grown wild asparagus) in glucose, fructose and sucrose levels and that the amount of these sugars differed considerably between the two ecotypes of grown A. acutifolius. However, Slatnar et al. (Reference Slatnar, Mikulic-Petkovsek, Stampar, Veberic and Horvat2018) reported that no significant differences were detected in sugar content among the different cultivars, which included six green and two purple-cultivated cultivars. Ferrara et al. (Reference Ferrara, Dosi, Di Maro, Guida, Cefarelli, Pacifico, Mastellone, Fiorentino, Rosati and Parente2011) suggested that soluble sugar content could vary greatly with many variables, which would be difficult to characterize quantitatively. The differences observed in our study between cultivated and wild asparagus could be ascribed to a concentration effect due to water-stress conditions under which they were grown, since according to Petropoulos et al. (Reference Petropoulos, Karkanis, Martins and Ferreira2018) sugars are a common tolerance mechanism of wild plants against stress. During the entire harvest season, glucose and sucrose were not significantly different between the harvest periods in both years; however, the patterns of fructose and total sugar differed only in the first year of the experiment, which tended to increase throughout the harvest season from the beginning to the end of the harvest period. These results are in agreement with those described previously by Anastasiadi et al. (Reference Anastasiadi, Collings, Shivembe, Qian and Terry2020), who reported that fluctuations in temperature during the harvest season appeared to coincide with changes in the biochemical profile of cultivated asparagus. This variation in sugar content can be explained by the fact that changes in the activities of sugar-metabolizing enzymes are influenced by the seasonal temperature during the harvesting season (Bhowmik et al., Reference Bhowmik, Matsui, Kawada and Suzuki2001). It was also seen that the content of fructose in fresh spears of cultivated and wild asparagus was higher than those of glucose and sucrose, this result was reported for only cultivated asparagus by Shou et al. (Reference Shou, Lu and Huang2007), Bhowmik et al. (Reference Bhowmik, Matsui, Kawada and Suzuki2001) and Bhowmik et al. (Reference Bhowmik, Matsui, Ikeuchi and Suzuki2002). To the best of our knowledge, this is the first report on seasonal variation in soluble sugars in wild asparagus.
In the current work, wild asparagus spears had a higher TPC (31% in 2018 and 37% in 2019) than cultivated asparagus spears. Data in the literature relating to the effects of species (cultivated and wild asparagus) on TPC are inconsistent. No difference was found in the TPC of spears developed from cultivated asparagus versus spears from wild asparagus (Palfi et al., Reference Palfi, Jurković, Ćosić, Tomić-Obrdalj, Jurković, Knežević and Vrandečić2017) but Ferrara et al. (Reference Ferrara, Dosi, Di Maro, Guida, Cefarelli, Pacifico, Mastellone, Fiorentino, Rosati and Parente2011), who compared fresh spears of wild asparagus with frozen spears of cultivated asparagus ‘UC800’, demonstrated that higher concentrations of TPC were present in wild asparagus. Similar results for TPC were reported by Nemzer et al. (Reference Nemzer, Al-Taher and Abshiru2020), who also observed higher TPC in wild purslane plants than in cultivated plants. Moreover, Kim and Yoon (Reference Kim and Yoon2014) reported that wild plants of L. indica contain higher amounts of polyphenols than cultivated plants. Additionally, some authors have stated that for cultivated asparagus, this trait is influenced by the genotype (Maeda et al., Reference Maeda, Kakuta, Sonoda, Motoki, Ueno, Suzuki and Oosawa2005; Slatnar et al., Reference Slatnar, Mikulic-Petkovsek, Stampar, Veberic and Horvat2018). In the present work, seasonal variations had an unstable effect on TPC, which showed minor fluctuations over the course of the season in both years. Higher TPC was observed in the mid-season harvest in the first year of the experiment; however, the early season harvest was better in the second year of the experiment. These results are in agreement with those described previously by Shou et al. (Reference Shou, Lu and Huang2007), Maeda et al. (Reference Maeda, Kakuta, Sonoda, Motoki, Maekawa, Suzuki and Oosawa2008) and Siomos (Reference Siomos2018), where the harvest period significantly affected the TPC in cultivated fresh asparagus spears (green and white). On the other hand, Siomos (Reference Siomos2018) indicated in his review that insignificant fluctuations in TPC were found during the harvesting period. This variation in TPC can be explained by the fact that TPC in spears is influenced by many environmental factors, the most important of which is temperature (Shou et al., Reference Shou, Lu and Huang2007). To the best of our knowledge, the current paper is the first report on TPC of wild asparagus in relation to seasonal variation.
The current research showed that wild asparagus spears had a higher AA (55% in 2018 and 92% in 2019) than cultivated asparagus spears. These results were similar to the findings of Ferrara et al. (Reference Ferrara, Dosi, Di Maro, Guida, Cefarelli, Pacifico, Mastellone, Fiorentino, Rosati and Parente2011), who reported higher AA levels in wild asparagus than in frozen cultivated asparagus. In contrast, Palfi et al. (Reference Palfi, Jurković, Ćosić, Tomić-Obrdalj, Jurković, Knežević and Vrandečić2017) compared cultivated and wild asparagus (collected from different locations) in terms of AA in the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay and reported that the AA of cultivated asparagus was higher than that of wild asparagus. These findings are common, and the tested samples showed different responses depending on the applied assay and extraction method (Petropoulos et al., Reference Petropoulos, Fernandes, Dias, Pereira, Calhelha, Di Gioia, Tzortzakis, Ivanov, Sokovic, Barros and Ferreira2020). The current paper also showed that seasonal variations had no effect on AA, where AA remained stable, with no significant differences between the harvest periods in either year. Contrary to our results, previous results showed that in white asparagus (A. officinalis), during the 40-day harvesting period, the AA measured using the FRAP method was the highest in the first half of the harvest period, and it was also significantly affected by the cultivars; however, the opposite result was observed for AA in the DPPH assay, peaking in the mid-season (Papoulias et al., Reference Papoulias, Siomos, Koukounaras, Gerasopoulos and Kazakis2009).
Conclusion
In conclusion, the study has shown that wild asparagus has better quality traits and biochemical compounds than asparagus cultivated for two consecutive years. Additionally, there was no significant difference in the TSS content, glucose, sucrose or AA during the harvest season in either year. Seasonal variations had unstable effects on colour, chlorophyll and TPC, which showed minor fluctuations over the course of the season. The patterns of fructose and total sugar differed only in the first year of the experiment and tended to increase throughout the harvest season from the beginning to the end of the harvest period. From the consumer's standpoint, the findings of our study are of great significance, as the recommended daily intake of nutrients can be covered by the consumption of less wild asparagus. Moreover, these findings may help breeders develop new cultivars with high quality, AA and TPC using wild asparagus.
Acknowledgements
The authors thank Professor Dr Serdar Gokhan Senol (Ege University, Faculty of Science, Department of Biology, Izmir, Turkey) for identifying the plant material.
Author contributions
OA and FS designed this study. OA, BT and FS conducted data collection. OA and FS performed the statistical analyses. OA, BS and FS drafted the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors
Conflict of interest
None.
Ethical standards
Not applicable.








