Management Implications
Panicum repens (torpedograss) is an invasive wetland grass in the southeastern United States. Glyphosate and imazapyr are commonly used for its control but are nonselective and may have non-target effects on valued native species such as Spartina bakeri (sand cordgrass). Sethoxydim is a grass-specific herbicide that was recently granted a 24(c) special local needs label for use in Florida. While it has shown some efficacy on P. repens and selectivity toward native dicot species, there is a need to optimize its use both to control P. repens and to limit damage to native grasses. Here, we evaluated the effect of application carrier volume and sethoxydim rate on P. repens and S. bakeri. Based on our results, managers may control or suppress P. repens by reducing carrier volumes from 935 L ha−1 to 187 or 37 L ha−1 with improved selectivity on S. bakeri. Overall, injury to S. bakeri was less than 40% across application rates and carrier volumes, suggesting that sethoxydim may provide some level of selectivity toward this native grass species. Managers may wish to use sethoxydim instead of glyphosate or imazapyr in areas where P. repens and S. bakeri are co-occurring, or as a follow-up treatment for regrowth of P. repens in areas where S. bakeri has been planted for restoration.
Introduction
Torpedograss (Panicum repens L.) is a perennial rhizomatous grass native to Africa and Eurasia that was introduced to the United States during the 1800s (Langeland et al. Reference Langeland, Cherry, McCormick and Craddock Burks2008). It was widely distributed throughout Florida for use as a wetland cattle forage in the early 1900s but escaped cultivation; by 1992, it was observed in more than 70% of public waters in the state (MacDonald et al. Reference MacDonald, Ferrell, Sellers, Langeland, Duperron-Bond and Ketterer-Guest2008). Panicum repens is found in both terrestrial and aquatic habitats, including lakeshores, marshes, and riparian systems (Wunderlin et al. Reference Wunderlin, Hansen, Franck and Essig2019). In aquatic systems, it grows along shorelines and in the water as floating mats, where it negatively impacts fish habitat and alters water quality (Hanlon and Brady Reference Hanlon and Brady2005; Hanlon and Langeland Reference Hanlon and Langeland2000). Panicum repens tolerates moderate levels of salinity and can be found in both freshwater and some brackish environments (Hossain et al. Reference Hossain, Ishimine, Akamine, Murayama, Uddin and Kuniyoshi1999; Prince and Macdonald Reference Prince and Macdonald2020; Smith et al. Reference Smith, Langeland and Hanlon1999).
Sand cordgrass [Spartina bakeri Merr.] is a native caespitose grass found primarily in Florida but also present in Georgia, South Carolina, and Texas. It is abundant in both fresh and brackish marsh systems, including those around Lake Okeechobee (Zhang et al. Reference Zhang, Denka, Deepak, Chen, Kutser, Collin and Warner2021), Indian River Lagoon (Schmalzer Reference Schmalzer1995), Ten Thousand Islands National Wildlife Refuge (Howard et al. Reference Howard, Day, Krauss, From, Allain and Cormier2017), and generally across coastal prairies of peninsular Florida (Wade et al. Reference Wade, Ewel and Hofstetter1980). Spartina bakeri is a robust perennial, growing to heights of 2 m, and can form large dense clumps. It is an important graminoid component in the fire ecology of Florida marshes (Schmalzer et al. Reference Schmalzer, Hinkle and Mailander1991). Although not well studied, S. bakeri co-occurs with prominent marsh invaders, including P. repens and old world climbing fern [Lygodium microphyllum (Cav.) R. Br.] (Hutchinson and Langeland Reference Hutchinson and Langeland2008). Therefore, S. bakeri is often a non-target concern for invasive plant control operations in marsh systems. While its response to fire and flooding have been studied (Schmalzer Reference Schmalzer1995), limited data exist on its tolerance to herbicides. Hutchinson and Langeland (Reference Hutchinson and Langeland2008) examined the impact of the herbicide metsulfuron on several native marsh species, including S. bakeri, and found a high degree of tolerance to rates commonly used for L. microphyllum control. This type of selectivity is highly desirable in invasive plant management. However, herbicide selectivity between invasive and native grasses is generally lacking.
Current management strategies for P. repens rely on nonselective, systemic herbicides such as glyphosate and imazapyr (Enloe and Netherland Reference Enloe and Netherland2017; Hanlon and Langland Reference Hanlon and Langeland2000). However, repeated applications are often necessary to achieve control of P. repens, and there are concerns about non-target damage to native species (Enloe and Netherland Reference Enloe and Netherland2017; Smith et al. Reference Smith, Shilling, Haller and MacDonald1993). This has led to increased interest in the use of graminicides such as sethoxydim, which was granted a 24(c) special local needs label for invasive grass management in aquatic habitats in Florida (Anonymous 2017). Sethoxydim inhibits fatty-acid synthesis by targeting the acetyl-coenzyme A carboxylase (ACCase) enzyme and is highly selective toward native dicot species (Burton et al. Reference Burton, Gronwold, Somers, Gengenback and Wyse1989; Enloe and Netherland Reference Enloe and Netherland2017). However, little is known about its selectivity toward non-target native grasses such as S. bakeri.
While greenhouse experiments suggest high efficacy of sethoxydim on P. repens (Enloe and Netherland Reference Enloe and Netherland2017), field evaluations have resulted in marginal control compared with glyphosate and imazapyr and required multiple applications per season, which significantly increases management costs (Enloe et al. Reference Enloe, Netherland and Lauer2018). In addition, sethoxydim may be less effective on P. repens under flooded conditions (Prince et al. Reference Prince, Quincy, Enloe, Macdonald and Netherland2019). Consequently, the product has not been widely adopted by managers in Florida. One possible way to increase sethoxydim efficacy is to alter its application technique. Foliar aquatic herbicide applications are largely made via airboat using high carrier volumes (average 935 L ha−1) (Haller Reference Haller, Gettys, Haller and Petty2020). However, spray retention on the target plant can be low when using high carrier volumes (Sperry et al. Reference Sperry, Mudge and Getsinger2022), and recent research suggests that reducing carrier volume to 187 L ha−1 increases efficacy of glyphosate, diquat, and 2,4-D on the aquatic plant water hyacinth [Eichhornia crassipes (Mart.) Solms] (Sperry and Ferrell Reference Sperry and Ferrell2021). Buhler and Burnside (Reference Buhler and Burnside1984) reported increased phytotoxicity of sethoxydim on forage sorghum [Sorghum bicolor (L.) Moench] and yellow foxtail [Setaria pumila (Poir.) Roem. & Schult.] with decreasing carrier volume. Likewise, Lassiter and Coble (Reference Lassiter and Coble1987) reported enhanced control of large crabgrass [Digitaria sanguinalis (L.) Scop.], fall panicum (Panicum dichotomiflorum Michx.), and goosegrass [Eleusine indica (L.) Gaertn.] with sethoxydim when carrier volume was reduced. However, other research suggests the effect of carrier volume on sethoxydim efficacy for other annual grasses may be influenced by environmental conditions (Harrison et al. Reference Harrison, Wax and Bode1986). Therefore, it would be very useful to also understand how these application characteristics influence potential sethoxydim injury on non-target native grasses. This is especially important for species like S. bakeri that are prominent in Florida marshes. Given these issues, our objective was to evaluate the effect of carrier volume and sethoxydim rate on P. repens control and S. bakeri response. Understanding this issue would potentially improve P. repens management within a restoration context.
Materials and Methods
A greenhouse experiment was conducted in 2020 at the University of Florida’s Center for Aquatic and Invasive Plants (CAIP) in Gainesville, FL (29.721542°N, 82.417300°W). The experiment was set up as a completely randomized design with four replications and a factorial arrangement of treatments plus a nontreated control (NTC). Factors consisted of carrier volume (935, 187, and 37 L ha−1) and sethoxydim rate (263, 526, and 1,052 g ai ha−1) (TIGR™ herbicide, SePRO, Carmel, IN, USA). Carrier volumes represented a typical high-volume spray-gun application, common broadcast application volume, and a low-volume application (Haller Reference Haller, Gettys, Haller and Petty2020). Sethoxydim rates represented 0.5X, 1X, and 2X the maximum label rate per hectare per application. Two independent runs were conducted. The first treatment was on June 8, 2020, and the second on June 25, 2020.
Panicum repens was established from stolons collected independently in early and mid-April for experimental runs 1 and 2, respectively, from a population growing in a pond at the University of Florida CAIP. Stolon fragments, 15 cm in length, were placed into tubs filled with water. Two weeks later, fragments that initiated new shoots and roots were selected for uniformity and planted into forty 3.8-L pots containing potting mix (Sun Gro® Metro-Mix 510, Sun Gro Horticulture, Agawam, MA, USA) amended with slow-release fertilizer (15-9-12, Osmocote® Plus, Scotts, Maryville, OH, USA) for each run. For S. bakeri, 1-yr-old established plants in 2.3-L pots were purchased from the Chiappini Farm Native Nursery (Hawthorne, FL, USA). A single plant from each pot was then subdivided equally into four separate pots. A total of 40 pots were planted in this manner for each run. Spartina bakeri plants were watered once daily via overhead irrigation, and P. repens plants were placed in 7-L tubs and subirrigated with well water. The water level in P. repens tubs was evaluated daily and maintained at 5 cm. Greenhouse temperatures were maintained at 29/24 C (max./min.) for the duration of the experiment. For both experimental runs, plants were allowed to establish for 8 wk before treatment.
Herbicide treatments were applied using a two-nozzle handheld boom and a CO2-pressurized backpack sprayer. The sprayer was calibrated to deliver 935, 187, or 37 L ha−1 using XR11005, XR11002, and XR1100067 nozzles (TeeJet®, Spraying Systems, Wheaton, IL, USA) at 276 kPa, respectively. Travel speed was adjusted to deliver the desired volumes. All treatments included a methylated seed oil (MSO) surfactant (Alligare MSO, Alligare, Opelika, AL, USA) at 0.25 % v/v. To reduce spray drift potential and cross-contamination, plants were sprayed outside the greenhouse and allowed to dry before being returned to greenhouse conditions.
Plants were visually evaluated for percent control (P. repens) or percent injury (S. bakeri) on a scale from 0% (no effect) to 100% (plant death) at 14, 28, and 42 d after treatment (DAT). After 42 DAT visual evaluations, aboveground biomass was harvested at the soil level, dried in a forced-air drying oven at 60 C for 5 d, and weighed. After 6 wk of regrowth, plants were evaluated for final oven-dried above- and belowground biomass. All data were subjected to two-way ANOVA, and means were separated using Fisher’s LSD test (α = 0.05) in the agricolae package in R (v. 3.6.1; Mendiburu Reference Mendiburu2019; R Core Team 2019). Percent control or injury data were arcsine square-root transformed to meet ANOVA assumptions, but back-transformed means are presented. Biomass data were normalized to the NTC and also arcsine square-root transformed. Biomass treatment means were also compared with the NTC by a paired t-test (α = 0.05).
Results and Discussion
Panicum repens data are presented as separate experimental runs due to an interaction between run and main effects for both visually evaluated control and biomass reductions. The differential response between runs was likely driven by plant maturity at the time of treatment; plants in run 1 were smaller than those in run 2 and had not yet developed an extensive network of rhizomes. No interactions between main effects (carrier volume and sethoxydim rate) were detected for P. repens control or biomass reduction data; however, main effects (sethoxydim rate and carrier volume) were independently significant (Tables 1 and 2).
Table 1. Visually evaluated Panicum repens percent control at 14, 28, and 42 d after treatment (DAT) as affected by main effects of sethoxydim rate and carrier volume from experiments conducted under greenhouse conditions in 2020.

a Means followed by the same letter within a column and main effect are not different according to Fisher’s LSD test (α = 0.05). Means followed by an asterisk (*) are not different from the nontreated control according to paired t-test (α = 0.05).
Table 2. Panicum repens biomass percent reduction as affected by main effects of sethoxydim rate and carrier volume from experiments conducted under greenhouse conditions in 2020.
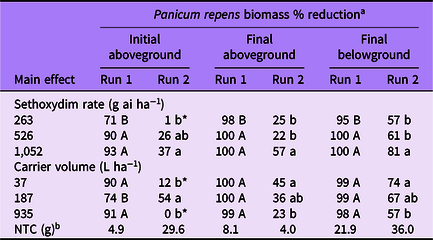
a Means followed by the same letter within a column and main effect are not different according to Fisher’s LSD test (α = 0.05). Means followed by an asterisk (*) are not different from the nontreated control according to paired t-test (α = 0.05). Mean pretreatment aboveground and belowground biomass values were 1.9 and 1.6 g for run 1; and 2.7 and 2.4 g for run 2, respectively.
b NTC, nontreated control. Biomass given in grams.
Panicum repens control increased with increasing sethoxydim rate in both experimental runs at 14 DAT (Table 1). In run 1, control increased from 84% to 99% for the 263 and 1,052 g ha−1 rates, respectively. In run 2, control increased from 2% to 41% for the same rates. Additionally, P. repens control at 14 DAT from the 263 g ha−1 rate in run 2 did not differ from the NTC. At 28 DAT, although the magnitude of control was different between runs, the pattern of sethoxydim activity was consistent. Panicum repens control in both runs was greatest in plants treated with 526 or 1,052 g ha−1 and the lowest sethoxydim rate in run 2 did not result in control levels different from that of the NTC. By 42 DAT in run 1, P. repens control was 99% or greater when at least 526 g ha−1 sethoxydim was applied. Conversely, P. repens control at 42 DAT in run 2 ranged from 19% to 65% and increased incrementally with sethoxydim rate.
Carrier volume effects on P. repens control in run 1 were only detected at 14 DAT, when maximum control was observed in the two lowest carrier volumes (Table 1). Panicum repens control was at least 94% by 28 and 42 DAT in run 1 regardless of carrier volume. However, carrier volume influenced P. repens control for run 2 for all data-collection dates. At 14 DAT, P. repens control was 6% to 8% greater in the two lowest carrier volumes than in treatments at 935 L ha−1. By 28 DAT, P. repens control was greatest in treatments applied at 37 L ha−1 (37% control). By 42 DAT, the control achieved with a carrier volume of 37 L ha−1 was more than double the control that was achieved at 935 L ha−1. In addition, control was greater in treatments applied at 187 L ha−1 than at 935 L ha−1.
When analyzed by sethoxydim rate, P. repens initial aboveground biomass reduction generally followed a similar pattern between runs, with the highest sethoxydim rate providing a greater biomass reduction than the lowest rate (Table 2). Final aboveground biomass was reduced at least 98% in run 1, indicating very little regrowth at all three sethoxydim rates. However, in run 2, sethoxydim at 1,052 g ha−1 reduced P. repens final aboveground biomass 57% compared with only 25% and 22% at 263 and 526 g ha−1, respectively. The final belowground biomass was reduced by 95% to 100% in run 1 and 57% to 81% in run 2.
The influence of carrier volume on P. repens biomass reduction differed between experimental runs (Table 2). In run 1, no meaningful differences were observed on smaller plants for final above- or belowground biomass. In run 2, the influence of carrier volume on larger, more established plants was not clear from the initial aboveground biomass harvest. This may be the result of variation in dead standing biomass at the time of initial harvest. However, the final aboveground and final belowground biomass harvests indicated that decreasing carrier volumes from 935 to 37 L ha−1 resulted in better P. repens control.
For S. bakeri data, there were no significant interactions found between main effects and experimental runs. Therefore, data were pooled across experimental runs. The lack of differences between experimental runs for S. bakeri contrasted with that observed for P. repens. This was likely due to the different belowground allocation strategies between caespitose and rhizomatous grasses. At 14 DAT, only the highest rate of sethoxydim resulted in injury (17%) that was different from that of the NTC; however, no differences in injury were observed among the treated rates (Table 3). At 28 DAT, all three sethoxydim rates resulted in S. bakeri injury and ranged from 20% to 37%. A generally similar pattern of injury was observed at 42 DAT with the highest two sethoxydim rates, which resulted in 14% to 22% greater injury than the lowest rate (Table 3).
Table 3. Visually evaluated Spartina bakeri percent injury at 14, 28, and 42 d after treatment (DAT) as affected by main effects of sethoxydim rate and carrier volume from experiments conducted under greenhouse conditions in 2020.

a Means followed by the same letter within a column and main effect are not different according to Fisher’s LSD test (α = 0.05). Means followed by an asterisk (*) are not significantly different from the nontreated control according to paired t-test (α = 0.05).
The influence of carrier volume on sethoxydim injury to S. bakeri did not follow a similar pattern to P. repens. At 14 DAT, S. bakeri injury was not different between any carrier volumes; however, treatments applied at 37 and 187 L ha−1 resulted in 12% and 13% injury, respectively, which was different from that of the NTC (Table 3). At 28 DAT, S. bakeri injury ranged from 26% to 32% among all three carrier volumes, with no differences among treated plants. By 42 DAT, injury ranged between 30% and 40%. Although these were all different from that of the NTC, there was no clear pattern of injury related to carrier volume.
Spartina bakeri biomass reduction was influenced by sethoxydim rate for all parameters measured. Sethoxydim at 1,052 g ha−1 reduced initial aboveground biomass by 51%, whereas 263 g ha−1 reduced biomass by 36% (Table 4). Final aboveground biomass was reduced by sethoxydim at the highest rate only. Final belowground biomass was reduced by all three sethoxydim rates and ranged from 24% to 47%, increasing with sethoxydim rate.
Table 4. Spartina bakeri percent biomass reduction as affected by main effects of sethoxydim rate and carrier volume from experiments conducted under greenhouse conditions in 2020.
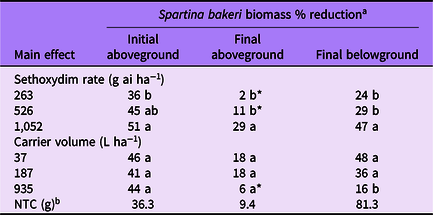
a Means followed by the same letter within a column and main effect are not different according to Fisher’s LSD test (α = 0.05). Means followed by an asterisk (*) are not different from the nontreated control according to paired t-test (α = 0.05). Mean pretreatment aboveground and belowground biomass values were 26.2 and 22.4 g, respectively.
b NTC, nontreated control. Biomass given in grams.
The influence of carrier volume on S. bakeri biomass varied by each biomass parameter measured (Table 4). All three carrier volumes resulted in 41% to 46% initial aboveground biomass reduction with no differences among treatments. For final aboveground biomass, the two lower carrier volumes resulted in an 18% reduction and were different from that of the NTC. The highest carrier volume did not differ in final aboveground biomass compared with that of the NTC. Final belowground biomass was reduced in all three carrier volume treatments compared with that of the NTC; however, reductions were greatest in the 37 and 187 L ha−1 treatments.
These results demonstrate several key points that contribute to our understanding of aquatic and wetland invasive grass management with sethoxydim. First, we observed significant differences in sethoxydim efficacy between the two runs, which was very likely plant size related. In the first run, the smaller, less-established P. repens plants were effectively controlled by all three sethoxydim rates. This was reflected in both the visually evaluated control and the above- and belowground biomass data. This supports the use of sethoxydim in treatment of incipient infestations before plants become well established where infested sites are likely to have greater plant diversity. In these scenarios, the use of traditional nonselective options, glyphosate and imazapyr, would likely cause excessive non-target damage. While aggressive monitoring would be required to implement this strategy, current maintenance control operations already in place for other invasive plants could be modified to monitor for new P. repens infestations in many public waters in Florida. Maintenance control has been well documented to be a more effective approach for management of nuisance and invasive floating plants in Florida (Joyce Reference Joyce1985), and incorporating selective P. repens control with sethoxydim could prevent new infestations from becoming problematic.
Second, when sethoxydim efficacy was less effective, we observed better control at the higher concentration tested, especially for belowground biomass. The current 24(c) sethoxydim label for Florida allows for single applications up to 526 g ha−1 (40 oz per acre) and spot treatments up to 5% v/v (Anonymous 2017). In our study, we found the 2X maximum labeled rate (1,052 g ha−1), which would result in a greater solution concentration similar to a spot treatment, was more effective than the 1X max label rate for reducing P. repens belowground biomass. Our data are in agreement with previous greenhouse work in which sethoxydim applied at 560 g ai ha−1 in the summer and fall reduced P. repens biomass by approximately 60% and 75%, respectively (Enloe and Netherland Reference Enloe and Netherland2017).
Third, we observed that reduced carrier volume improved P. repens control for sethoxydim in terms of final above- and belowground biomass harvests. While high-volume handgun applications are widely used for aquatic plant control, our work provides some support for reducing carrier volume, similar to other recent aquatic studies (Sperry and Ferrell Reference Sperry and Ferrell2021). Reducing carrier volume increases herbicide concentration of the spray solution, which in turn drives increased herbicide uptake and translocation, especially for systemic herbicides (Knoche Reference Knoche1994). Previous studies that evaluated terrestrial grass weeds also noted increased efficacy and consistency when sethoxydim was applied in lower carrier volumes (Lassiter and Coble Reference Lassiter and Coble1987; McMullan Reference McMullan1995). Furthermore, these data confirm that sethoxydim efficacy could be maintained in aerial or ground broadcast applications that utilize much lower carrier volumes; however, the current label instructions require applications to be made at a minimum of 140 L ha−1 (Anonymous 2017).
Fourth, we observed some tolerance of S. bakeri to sethoxydim at all concentrations tested. While absolute sethoxydim selectivity, which is typical for dicots that have an insensitive ACCase enzyme, was not observed, we did observe injury of 45% or less. This would likely be better than the nonselective herbicides glyphosate and imazapyr, as has been shown for other species (Enloe and Netherland Reference Enloe and Netherland2017; Hedge et al. Reference Hedge, Kriwoken and Patten2003; Mateos-Naranjo et al. Reference Mateos-Naranjo, Redondo-Gomez, Cox, Cornejo and Figueroa2009, Reference Mateos-Naranjo, Cambrolle, Lomas, Parra and Redondo-Gomez2012; Patten Reference Patten2002). We did not test these in this study, as we chose to focus exclusively on sethoxydim concentration and carrier volume.
Finally, altered carrier volume did not have a consistent effect on S. bakeri injury, initial aboveground biomass, and final aboveground biomass. While these results were promising, we did observe the two lower carrier volumes reduced belowground biomass significantly more than the highest application volume. This pattern was also somewhat observed, albeit to a greater extent, in P. repens rather than the non-target S. bakeri. Future research should incorporate field studies that examine the carrier volume–sethoxydim concentration relationship for P. repens control and nontarget native grasses, including S. bakeri.
Acknowledgments
The authors would like to thank J. P. Keller and Conrad Oberweger for technical assistance in conducting experiments. No conflicts of interest have been declared.







