Until recently, only three species of Rubus were recognized as naturalized along the West Coast of the United States. These are: (1) R. armeniacus Focke (=R. discolor auct. non Weihe & Nees and R. procerus auct. non P. J. Müll. ex Boulay), commonly known as Armenian, Himalaya, or Himalayan blackberry; (2) R. laciniatus Willd., commonly known as cutleaf blackberry; and (3) R. ulmifolius Schott (=R. discolor Weihe & Nees), commonly known as elmleaf blackberry (Alice Reference Alice2015; DiTomaso and Healy Reference DiTomaso and Healy2007). Using primarily molecular characteristics, Clark et al. (Reference Clark, Evans and Jasieniuk2013) substantiated the presence of these species and documented the establishment of two others: R. vestitus Weihe, also described earlier from Salem, OR, by Bailey (Reference Bailey1945); and a cryptic, but genetically distinct species from northern California, Oregon, and Washington, which they identified as R. anglocandicans A. Newton. Because the putative R. anglocandicans is similar in appearance to and is frequently sympatric with R. armeniacus, it is also generally known as Armenian blackberry. According to Clark et al. (Reference Clark, Evans and Jasieniuk2013), the most common naturalized species along the Pacific coast of the United States is R. armeniacus, ranging in distribution from California to Washington State. The greatest diversity among these species was at a site near Eugene, OR (Clark et al. Reference Clark, Evans and Jasieniuk2013).
Box 1 Management Implications
Identifying invasive blackberry species along the Pacific coast of North America makes it possible to determine the importance of each species in the invasion process. Clarifying morphological differences facilitates field identification, thus making it possible to now map distributions of each, to determine the relative importance of each, to measure shifts in population densities over time, and, although Rubus armeniacus and R. praecox are sympatric, to clarify subtleties in habitat and environmental preferences that may affect strategies for management. Determination that the two species differ in susceptibility to the rust disease caused by Phragmidium violaceum affects implementation of biological control measures. The fact that the rust disease is not effective on R. armeniacus and requires application of other management strategies. Also, development of biological control of this species will necessitate searches for agents other than P. violoaceum in its native range.
Discovery of R. anglocandicans was unexpected (Clark et al. Reference Clark, Evans and Jasieniuk2013). The name R. anglocandicans was applied by Clark et al. (Reference Clark, Evans and Jasieniuk2013) to these newly recognized U.S. accessions because they clustered with Australian accessions reported under this specific epithet (Evans and Weber Reference Evans and Weber2003). The name for Australian populations was assigned on the basis of banding patterns from an M13/HaeIII DNA digest protocol that were identical to those of R. anglocandicans from the United Kingdom (Evans and Weber Reference Evans and Weber2003). Although the U.S. and Australian accessions were found to be similar on the basis of molecular data, they did not cluster with accessions of R. anglocandicans from the United Kingdom in the study by Clark et al. (Reference Clark, Evans and Jasieniuk2013), leading to the conclusion that these two groups (Australian/U.S. vs. UK) were from different asexual lineages. Evans and colleagues (Evans and Weber Reference Evans and Weber2003; Evans et al. Reference Evans, Symon, Whalen, Hosking, Barker and Oliver2007) described difficulty in assigning the name “anglocandicans” to their accessions. Despite this, the name R. anglocandicans was applied by Clark et al. (Reference Clark, Evans and Jasieniuk2013) to the U.S. material.
European blackberry is invasive in Chile, Australia, and the United States. For this reason, biological control using Phragmidium violaceum (Schultz) G. Winter, the cause of a rust disease, was pursued both in Chile (Oehrens and Gonzalez Reference Oehrens and Gonzalez1974; Rejmánek Reference Rejmánek2015) and in Australia. Two strains were introduced originally into Australia, one illegally in 1984 and a second after a complete risk assessment (Mahr and Bruzzese Reference Mahr and Bruzzese1998). Variability in disease response was noted within Australian “European blackberry” after introduction of the two rust fungal strains (Evans et al. Reference Evans, Jones and Roush2005). Further investigation revealed that “European blackberry” in Australia is a complex, including “at least 15 polyploid agamospecies… and one diploid, sexual species” (Evans et al. Reference Evans, Symon and Roush1998, Reference Evans, Symon, Whalen, Hosking, Barker and Oliver2007). Rubus anglocandicans was considered to be the most widespread of these (Evans and Weber Reference Evans and Weber2003). To improve management of European invasive blackberry in Australia, eight additional strains of the rust fungus were released in 2004 (Morin et al. Reference Morin, Aveyard, Batchelor, Evans, Hartley and Jourdan2006, Reference Morin, Evans, Jourdan, Gomez and Scott2011).
Based upon the Australian experience and considering the magnitude of the invasive blackberry problem along the Pacific coast of North America, P. violaceum has also been of interest for biological control in the United States (Bennett Reference Bennett2007; DiTomaso and Healy Reference DiTomaso and Healy2007; Peters Reference Peters2012). A strain of P. violaceum was discovered in Oregon in 2005 (Osterbauer et al. Reference Osterbauer, Trippe, French, Butler, Aime, McKemy, Bruckart, Peerbolt and Kaufman2005), before formal U.S. evaluations had been initiated. Anecdotally, observations from 2004 and surveys in 2005 revealed severe dieback and death of blackberry that was attributed to the rust disease (Osterbauer et al. Reference Osterbauer, Trippe, French, Butler, Aime, McKemy, Bruckart, Peerbolt and Kaufman2005). There was also evidence of diseased R. laciniatus at that time, including in commercially important ‘Thornless Evergreen’ and ‘Everthornless’ cultivars of R. laciniatus (Johnson and Mahaffee Reference Johnson and Mahaffee2010; Osterbauer et al. Reference Osterbauer, Trippe, French, Butler, Aime, McKemy, Bruckart, Peerbolt and Kaufman2005). More recently, rust disease was described on both R. armeniacus and R. laciniatus in Canada (Callan et al. Reference Callan, Wall, Dale and Joshi2011).
As in Australia, both variability in disease severity and incidence were noted in the United States by Johnson and Mahaffee (Reference Johnson and Mahaffee2010), who speculated about a “minor biotype” of R. armeniacus that was highly susceptible to P. violaceum in Oregon. They identified the remainder, i.e., “the more predominant biotype,” as not susceptible. Objectives of this research were to evaluate the effects of P. violaceum from Oregon on invasive Rubus species and to consider its potential for biological control of invasive blackberry along the West Coast of the United States
Materials and Methods
Clarifying susceptibility of introduced Rubus species was accomplished in two research thrusts. The first involved field surveys, which led to the second phase, i.e., the collection of plant material for greenhouse inoculation and study. It became necessary to identify specimens morphologically in order to fully understand differences noted in disease response in both the field and the greenhouse.
Field Survey
Between 2005 and 2009, field observations were made periodically at blackberry sites selected for the most part on the basis of symptomatic plants but also including some identified by collectors as “resistant” or not diseased. Specimens from these sites were sent to the USDA, ARS, Foreign Disease–Weed Science Research Unit (FDWSRU) for processing. This resulted in accumulation of several living blackberry clones and isolates of the pathogen, including the establishment of the disease under greenhouse conditions. Greenhouse inoculations were initiated in 2009.
To more fully understand this disease in Oregon within populations of feral blackberry, a random sampling was made in 2010 that included visits to more than 30 sites during the week of August 2. The area surveyed was west of the Cascade Mountains in Oregon, and ranged from Portland to Ashland (Figure 1), i.e., the Willamette Valley and sites along the Pacific coast of Oregon. The objective of site visits was to collect, if possible, one diseased and one “healthy” (i.e., nonsymptomatic) plant specimen at each location. Records for each site included GPS coordinates, text description of the location, notes on disease incidence, and photographs. Each plant specimen was trimmed at the field site, and the cut end was put into an Aquatube (Syndicate Sales, Kokomo, IN 46901) filled with water. Living specimens were then put into plastic bags and shipped overnight to the FDWSRU. Upon receipt, packages were opened and processed in the microbial-containment greenhouse and laboratory facility at the FDWSRU. Collection numbers were assigned to both plant and pathogen acquisitions. Pathogen samples were stored in a −80 C ultracold freezer. Plant samples were treated with a rooting hormone (Hormex® Liquid Concentrate plus Vitamin B1, Brooker Chemical Corporation via Maia Products, Chatsworth, CA 91313), put into rock wool (OASIS® Grower Solutions, Smithers-Oasis North America, Kent, OH 44240), and set into a misting tent for rooting. Canes of specimens rooted in containment were cut for removal following a protocol approved by regulators from the USDA, Animal and Plant Health Inspection Service (APHIS). It involved surface sterilization of cuttings (15% bleach for 5 min), removal from containment, and transfer to a conventional greenhouse for rooting and propagation. In addition to field samples from Oregon, one accession of R. praecox (Oehrens and Gonzalez Reference Oehrens and Gonzalez1974; Rejmánek Reference Rejmánek2015) was supplied by L. Ciampi (Univ. Austral de Chile, Valdivia), and two accessions each of R. praecox and R. armeniacus were supplied from the Czech Republic by M. Sochor and B. Trávníček.

Figure 1 Survey routes for invasive blackberry in Oregon, 2009–2015, showing locations for plants that were not diseased (“0,” Rubus armeniacus) or diseased by Phragmidium violaceum (“1,” R. praecox s. lat.) in the field and/or in greenhouse tests. Not shown are locations for R. laciniatus or R. vestitus.
Rooted specimens for the production of test canes were transplanted into 15-cm clay pots filled with a standard artificial soil mix of peat (41%), bark (11%), perlite (23%), vermiculite (23%), sand (2%), and Micromax® trace minerals (Scotts Miracle-Gro, Marysville, OH 43040). Herbarium material was not collected in 2010.
Field sites were revisited by colleagues from the Oregon Department of Agriculture in 2011 and 2012, who noted status of disease and provided additional pathogen acquisitions and herbarium specimens. In 2013, 2014, and 2015, collecting trips were made to sites visited originally in 2010 and selected on the basis of results from greenhouse inoculations. This enabled confirmation of earlier findings, the development of additional notes, photographic records, and the collection of additional plant and pathogen samples for tests (locations illustrated in Supplemental Figure 1). Plant material was also specifically collected for herbarium records (Supplemental Figure 2a–i) and for both morphological characterization and measurement, e.g., of leaves (Figure 2), which facilitated identification of species. In 2014 and 2015, petal samples were collected on cellophane tape that was put onto index cards (Supplemental Figure 3). Petals were measured for length and width.

Figure 2 Drawings of leaf and leaflets, modified from Weber (Reference Weber1995), illustrating measurements taken from herbarium specimens.
Greenhouse Inoculations
The greenhouse investigation was initiated in 2009, when three isolates were established and increased after artificial inoculation. The isolate FDWSRU #09-020, from very heavily diseased blackberry along the Elk River (Figure 3), was used for most of the greenhouse studies. More recently, inoculations involved FDWSRU #09-010, collected at the Langlois Store site.

Figure 3 Heavily diseased leaf of invasive blackberry near the Elk River in Oregon. This isolate was used in the majority of greenhouse tests for susceptibility to disease.
Plant accessions were increased by periodic rooting, as described, and maintained in a conventional, locked greenhouse in 15-cm clay pots, according to APHIS permit. For disease-response studies, three to five canes from as many as five accessions were cut and placed in water in 1- or 2-L flasks. Each flask (a “bouquet”) contained a representative set of accessions (replication within inoculation), and there were three to five flasks per inoculation (Figure 4). Canes of a known susceptible accession from Lincoln City, OR, were included as a positive control in each bouquet. Attempts were made to inoculate each accession on at least three separate occasions (=3 repetitions).

Figure 4 Flasks of canes with test blackberry accessions (bouquets) for inoculation by Phragmidium violaceum.
Inoculum was used either fresh or after ultracold storage. A suspension of urediniospores in water was sprayed onto bouquets at the rate of 5 mg flask−1, and inoculated canes were given two 16-h dew treatments at 18 C in the dark separated by an 8-h period at laboratory conditions outside of the dew chambers. Flasks were placed on a bench in a 25 C greenhouse, and canes were observed for symptoms. Data were recorded for pustules on the three most-diseased leaves and for both the total number of inoculated leaves and the number of symptomatic leaves. Incidence, i.e., proportion of diseased leaves, was calculated.
Plant Identifications
Identifications were based on morphological characteristics as described by Edees and Newton (1998), Weber (Reference Weber1972, Reference Weber1995), Zielinski (Reference Zielinski2004), and from the long-term taxonomical experience of the Czech coauthors with bramble populations in western and central Europe. Translations of German keys were provided by Christine Dieckhoff (USDA, ARS, Newark, DE). Identifications were based upon data collected from the field, from herbarium sheets, and from petal cards. Herbarium sheets have been submitted to the L. H. Bailey Hortorium at Cornell University (BH), the Ada Hayden Herbarium at Iowa State University (ISU), the Oregon State University Herbarium (OSC), the University of California at Davis Herbarium, and the Palacký University herbarium (OL), Olomouc, Czech Republic.
Flow Cytometry
Ploidy level of invasive Rubus from Oregon was determined using flow cytometry. Accessions assessed were one specimen of R. laciniatus (072407-1), three specimens of R. armeniacus (080709-3, 080210-4, and 080310-1), and five specimens of R. praecox s. lat. (080410-1, 080410-3, 080510-9, 080510-12, and 071315-4). These accessions represent a range of locations in Oregon (Table 1), and those of R. praecox in particular represented a range of leaf morphological characteristics within these accessions.
Table 1 Locations in Oregon (except where noted) that were surveyed and rust disease caused by Phragmidium violaceum observed both in the field and after artificial greenhouse inoculation.

a Field observations and identifications between 2009 and 2015.
b Abbreviations and symbols: A, Rubus armeniacus; P, R. praecox s. lat.; L, R. laciniatus; V, R. vestitus. Disease response: minus sign (−), no disease; plus sign (+), disease; triple plus sign (+++), heavily diseased; DNG, did not grow (accession lost). Two specimens were collected, one not symptomatic in the field (+/−). Both accessions were susceptible in greenhouse tests (+/+).
c Accessions that were collected, grown, and tested.
Ploidy-level determinations were based on the relative fluorescence of stained nuclei using flow cytometry measurements of fresh leaves from a BD Accuri C6 (BD Biosciences, Franklin Lakes, NJ 07417) flow cytometer. As an internal standard, Solanum lycopersicum ‘Stupické polní rané’ (somatic cell DNA content 2C=1.96 pg; Doležel et al. Reference Doležel, Binarová and Lucretti1989) was used. Leaf tissues of the sample and standard were chopped together with a razor blade in 0.5 ml LB01 buffer (Doležel et al., Reference Doležel, Binarová and Lucretti1989; 15 mM Tris, 2 mM EDTA, 0.5 mM spermine tetrahydrochloride, 80 mM KCl, 20 mM NaCl, 0.1% Triton X-100, 30 g L−1 PVP40, and 550 μl L−1 2-mercaptoethanol [pH=8.0]). The suspension was filtered through a 42-μm nylon mesh and stained with 20 μl of propidium iodide. At least 3,000 particles were measured within the size limits of the sample and the standard only. BD Accuri C6 (BD Biosciences) software was used to calculate peak positions and coefficients of variation (CV). The highest CV value for accepted peaks was approximately 5.0%. For ploidy-level calibration, genotypes of R. moschus (2n=14; chromosomes counted by Krahulcová and Holub Reference Krahulcová and Holub1997; documented by herbarium specimen OL no. r266/11 collected by Trávníček) and R. bifrons Vest (2n=28; counted by Tesařová Reference Tesařová2012; documented by OL specimen Dus2) were also measured.
Statistical Analyses
The mean incidence for each accession within a replication from greenhouse inoculations was used for data analysis. Results from at least two or, for the most part, three repetitions were included in the analysis of each accession. Incidence data were analyzed using Proc GLM (SAS Institute, Cary, NC 27513) to test the model: incidence=species. Data were tested for randomness and normality using Proc UNIVARIATE before analysis and transformed, if necessary. LSMeans were calculated from the GLM analysis and considered statistically significantly different if P≤0.05, based upon probability of differences (PDIFF) in SAS. In addition, the variable incidence was converted into a disease variable, with values of either “0” (=no disease) or “1” (=diseased) assigned to each accession within a replication. These data were analyzed using Proc GLIMMIX in SAS, modeled as a random variable from a beta distribution, and analyzed as a one-factor generalized linear model with species as the factor. Assumptions of the models were checked. Comparisons were done with Sidak-adjusted P-values so that the experiment-wise error was 0.05.
Results and Discussion
Plant Morphology, Identification, and Species Distribution
Four species of introduced Rubus, i.e., R. armeniacus, R. laciniatus, R. vestitus, and the cryptic species R. praecox s. lat., were found in Oregon during this survey. Two of these species, i.e., R. laciniatus and R. vestitus, were easily distinguished morphologically and were not particularly common. Rubus laciniatus was found at four of 33 sites, and R. vestitus was found at one (Table 1). Clark et al. (Reference Clark, Evans and Jasieniuk2013) reported that R. ulmifolius occurred only in California, and it was not found during the present survey in Oregon, possibly due to its preference for an oceanic climate (Kurtto et al. Reference Kurtto, Weber, Lampinen and Sennikov2010; B Trávníček and M Sochor, personal observations).
The other two species were found to be very common, widely distributed, and invasive throughout Oregon west of the Cascade Mountains (Table 1; Figure 1). Both belong to the same tetraploid complex of Rubus series Discolores. Although R. armeniacus was described from central Europe as an invasive crop, it originated in the eastern Transcaucasian region (Focke Reference Focke1910; Sochor 2016). Rubus armeniacus is probably a strictly asexual species with low intraspecific morphological variability. In contrast, R. praecox s. lat. is a European taxon that includes several very close, facultatively asexual lineages, which are usually grouped under the species name R. praecox (e.g., see Kurtto et al. Reference Kurtto, Weber, Lampinen and Sennikov2010). Moreover, poor type material has led to many recent discussions about the proper application of this name (e.g., van de Beek Reference van de Beek2014). Improved taxonomic understanding of this group is likely to develop in the near future.
Values of relative fluorescence of stained nuclei from the flow cytometry protocol of specimens from Oregon indicated tetraploid level of all R. armeniacus (three accessions; mean relative fluorescence ratio sample:standard RF=0.744±0.0016 [SE]), all R. praecox s. lat. (five accessions; RF=0.765±0.0017), and R. laciniatus (one accession; RF=0.738). These results are in accordance with values reported for these species from Europe (Krahulcová et al. Reference Krahulcová, Trávníček and Šarhanová2013; M Sochor, unpublished data). Unfortunately, no ploidy data have been reported for R. anglocandicans to date.
On the basis of morphological features that separate the two species, R. armeniacus was recorded in 24 (or 73%) of the 33 locations, R. praecox was recorded in 27 (or 82%) of the locations, and of these, both species occurred at 19 (or 58%) of the locations (Table 1). These results suggest both species are nearly equal in occurrence, very common throughout the range of the survey, and frequently sympatric.
Rubus armeniacus was distinguished by large pink (occasionally very pale pink, almost white) flowers that have large petals (Table 2; Figure 5), very long stamens (up to twice as long as styles), straight prickles on the inflorescence axis (Figure 6, left), and terminal primocane leaflets that are large and very broadly elliptical to nearly round (Figure 5), measuring 9.6±0.5 cm long (mean ± confidence interval (c.i.), P=0.05; Table 3). Inflorescences are usually large and loose (Figure 6, left). The terminal leaflet on primocanes often is cuspidate. Plants are large, often >2-m tall, robust, and with arching stems.

Figure 5 Rubus armeniacus from Monroe Park, OR, growing near R. praecox (illustrated in Figure 7): (A) single pink flower with very long stamens, (B) inflorescence axis with straight prickles and pink flowers showing long stamens, and (C) three leaves showing characteristic leaflet arrangement and morphology.

Figure 6 Herbarium sheets of inflorescences of Rubus armeniacus (left) and R. praecox s. lat., both from Monroe Park, OR.
Table 2 Least-square means of petal measurements (mm) from Rubus armeniacus, R. praecox s. lat., and R. vestitus collected in Oregon.

a Means followed by the same letter are not statistically different (P=0.05).
Table 3 Least-square means (LSMs) and ranges of terminal leaflet measurements (cm) from Rubus armeniacus, R. praecox s. lat., and R. vestitus collected in Oregon.

a Means followed by the same letter are not statistically different (P=0.05).
The second species, very similar in gross morphology to R. armeniacus, was identified as R. praecox s. lat., having medium-sized white flowers as suggested by petal size (Table 2), buds that are sometimes slightly pinkish, stamens only slightly longer than style, elliptical to broadly elliptical terminal leaflets that are smaller, 7.7±0.5 cm long (c.i., P=0.05; Table 3), and curved prickles on the inflorescence axis (Figures 6, right, 7). Inflorescences are moderate in size and more compact (Figure 6, right) than those of R. armeniacus (Figure 6, left).
Distinct morphological differences were noted between R. praecox and R. armeniacus in this study (Figures 5–8). Petals (Table 2) and terminal leaf sizes (Table 3) were significantly smaller for R. praecox compared with R. armeniacus. Even so, leaf size was not a particularly useful diagnostic in the field, considering overlap in the range of terminal leaflet dimensions (Table 3). There was tendency for all leaflets of R. praecox to be more narrowly elliptical than those of R. armeniacus (Table 3; Figure 8). Differences in prickle morphology in the inflorescences (or infructescences) and flower size and morphology, particularly relating to petal size and the length of stamens, were very useful in the field.

Figure 7 Rubus praecox s. lat. from Monroe Park, OR, growing near R. armeniacus (illustrated in Figure 5): (A) two flowers with stamens of moderate length, (B) stem of inflorescence showing chacteristic curved prickles and flower and leaf morphology, and (C) a characteristic leaf with symptoms of Phragmidium violaceum rust disease.

Figure 8 (A) Field specimens and (B, C) herbarium sheets of diseased Rubus praecox s. lat. (left) and R. armeniacus (right), collected within 20 m of each other.
Disease Responses
Results of the field survey and artificial greenhouse inoculations with P. violaceum revealed clear differences in susceptibility of invasive Rubus species in Oregon. The fact that R. armeniacus, of the two cryptic species, is not susceptible (Tables 1, 4; Figure 8) was unexpected. Similar differences were noted in tests with a Chilean accession of R. praecox, which was susceptible, and with four accessions from the Czech Republic, two of R. armeniacus that were not susceptible and two of R. praecox that were (Table 1). Similar results were reported from Germany by Schön (Reference Schön2014), who found that R. bifrons, R. laciniatus, and R. praecox were susceptible to rust disease caused by P. violaceum. It was also learned that R. armeniacus is not infected by P. violaceum in Germany (M Schön, personal communication).
Table 4 Means and mean comparisons of disease incidence (% infection) after greenhouse inoculations of Rubus armeniacus, R. praecox s. lat., and R. laciniatus with Phragmidium violaceum from Oregon.
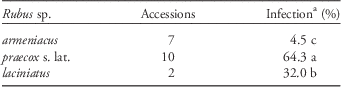
a Means followed by the same letter are not statistically different at the 0.05 significance level.
That R. laciniatus is susceptible was known from previous reports (Callan et al. Reference Callan, Wall, Dale and Joshi2011; Johnson and Mahaffee 2010; Osterbauer et al. Reference Osterbauer, Trippe, French, Butler, Aime, McKemy, Bruckart, Peerbolt and Kaufman2005; Schön (Reference Schön2014), and such was confirmed in greenhouse tests (Table 4). Rubus vestitus was also found to be diseased in the field, but it was not tested in greenhouse studies (Tables 1, 4). The lack of disease as a diagnostic for R. armeniacus is useful, but it can be variable, including absent, on R. praecox from year to year. Thus, morphological features, rather than disease, should be used for field identifications.
Rubus anglocandicans.
One issue is the correct name for the U.S. species identified by Clark et al. (Reference Clark, Evans and Jasieniuk2013) as “cryptic,” and the presence of which was supported by results described in this paper. It has been identified as R. praecox s. lat. in this study, and it is likely the same species as that named R. anglocandicans by Clark et al. (Reference Clark, Evans and Jasieniuk2013), a name that was applied due to the fact that it clustered with Australian material named R. anglocandicans on the basis of M13/HaeIII DNA digest banding patterns (Evans and Weber Reference Evans and Weber2003). There are considerations that support R. praecox s. lat. as the correct name for these invasive bramble populations. In both Evans and Weber (Reference Evans and Weber2003) and Evans et al. (Reference Evans, Symon, Whalen, Hosking, Barker and Oliver2007), accounts of uncertainty in reaching their determination of R. anglocandicans were presented. Clear differences noted between Australian and UK accessions of R. anglocandicans included lack of hairs on stems, terminal leaflets that are larger and more broadly elliptical (resembling that of R. armeniacus), and relatively longer petioles, among other features. Very clear difference was noted, particularly in petal size (Table 5), which was much larger than that of R. anglocandicans (Edees and Newton Reference Edees and Newton1988). Considering these factors, R. praecox s. lat. is the correct name for these blackberries.
Table 5 Petal measurements (mm) from Rubus armeniacus, R. praecox s. lat., and R. vestitus from the present study compared with data from the literature.

a Entries showing only a range are about petal length. Outliers in dimensions are set off by parentheses.
b Identified as R. procerus in the work cited.
Rubus armeniacus.
Identity of R. armeniacus as invasive along the Pacific coast of the United States and Canada is strongly supported by reports in the literature. Ceska (Reference Ceska1999) first applied this name on the basis of determinations by European expert batologists, who indicated that those epithets applied previously, i.e., R. discolor and R. procerus, were incorrect. Clark et al. (Reference Clark, Evans and Jasieniuk2013) also found that U.S. accessions of R. armeniacus clustered with those of German origin in a molecular study of invasive U.S. blackberries.
Conclusions
Important new perspectives developed concerning identity of invasive Rubus along the North American Pacific coast, the importance of R. praecox in Oregon, and the differential response of these species to a blackberry rust disease. Clark et al. (Reference Clark, Evans and Jasieniuk2013) reported that R. armeniacus was the more common of the two species in their survey of California, Oregon, and Washington, but in the present study, R. armeniacus and R. praecox appeared essentially equal in proportion (Table 1; Figure 1). Shift in the predominance of one Rubus species over another was reported previously by Bammi and Olmo (Reference Bammi and Olmo1966), who described a shift from the predominance by R. laciniatus in the early 1930s to that of R. procerus (=either R. armeniacus or R. praecox) by the 1960s. Results of this study raise a question about the relative importance of R. armeniacus vs. R. praecox and which will eventually predominate along the West Coast of North America.
A question also arises about effect of the rust disease as an environmental stress factor. Will disease tip the balance in favor of R. armenicacus as the species that predominates? Research in the future can examine the importance and role of each of these species as invasives and determine the effect of rust disease on the density of each.
Although separation of R. armeniacus from R. praecox in the field was generally clear, morphological variants were encountered that were difficult to delimit. This raises the possibility for additional species, not yet recognized, and substantiates the need for additional tools that facilitate blackberry identification. The possibility that variability is related to some sort of crossing event, or hybridization, has been described (Alice Reference Alice2002; Alice and Campbell Reference Alice and Campbell1999), and the importance of hybridization, including development of commercially important varieties within Rubus, was discussed by Waugh et al. (Reference Waugh, Van De Ven, Phillips and Powell1990). Clark and Jasieniuk (Reference Clark and Jasieniuk2012) more recently presented evidence for natural crosses between native Rubus ursinus Cham. & Schldl.×Rubus pensilvanicus Poir., introduced into California from the eastern United States, and R. ursinus×invasive, exotic R. armeniacus. Additional support for naturally occurring hybridization comes from investigation of a blackberry that was intermediate in form between R. laciniatus and “R. procerus” (Bammi and Olmo Reference Bammi and Olmo1966).
Species identification and effects of disease on invasive blackberries need to be included in broader ecological studies. Previous findings about blackberry and ecosystem effects can be substantiated or improved, including, e.g., a study on population demographics as affected by growth and reproduction of native and noninvasive Rubus species (Lambreche-McDowell and Radosevich 2005); effects of light and soil on blackberry growth, studied earlier for R. armeniacus by Caplan and Yeakley (Reference Caplan and Yeakley2006); and functional morphology of invasive blackberries and noninvasive species, described also by Caplan and Yeakley (Reference Caplan and Yeakley2013) for R. armeniacus.
Acknowledgments
Technical support was provided by Sara Meyers and Alyssa Kloos. Reviews and assistance with graphics by colleagues at the USDA, ARS, FDWSRU, is gratefully acknowledged. Christine Dieckhoff (formerly USDA, ARS, Newark, DE) generously made translation from the German of Rubus keys by Weber and Mary Camp (USDA, ARS, Beltsville, MD) provided statistical advice and computations. Generous support in the form of consultation, plant accessions (healthy and diseased), and encouragement was provided by the Oregon Department of Agriculture, Salem, OR (Tim Butler, Eric Coombs, Carri Pirosko, Amy Peters, Ken French); by Mark Wiederlechner (Iowa State University, Ames); Jean Shepard (University of California, Davis, Herbarium); Lindsay Clark (University of Illinois, Urbana–Champaign); Gerry Moore (USDA, Natural Resources Conservation Service); and Walt Mahaffee (USDA, ARS, Corvallis, OR). Support was also provided through an internal grant from Palacký University, Olomouc, Czech Republic (IGA_PrF_2014-001, IGA_PrF_2015_001) and Grant No. LO1204 (Sustainable development of research in the Centre of the Region Haná) from the National Program of Sustainability I, Ministry of Education, Youth and Sports of the Czech Republic. Scans of herbarium specimens were provided by Martina Oulehlová, Palacký University, Olomouc, Czech Republic. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2017.12















