Management Implications
The invasive winter annual grass Bromus tectorum (downy brome) has invaded vast expanses of sagebrush-grassland in western North America, and the fine fuel associated with invasion increases the frequency of wildfire such that native plants struggle to persist. Recent research suggests that B. tectorum invasion may expand across an even larger portion of the U.S. Intermountain West in the absence of effective and proactive management.
Imazapic is widely used to manage B. tectorum, but control often declines after 1 yr, and reinvasion is typical. Several trials have demonstrated that the newer herbicide indaziflam can selectively control annual grasses for 3 or more years, and past studies indicate that B. tectorum seedbanks are relatively short-lived in the field (≤5 yr). Thus, consecutive years of control with indaziflam may deplete B. tectorum seedbanks and increase the duration of control, but it is unclear whether this will require multiple applications.
A single indaziflam treatment at intermediate and high rates (73 and 102 g ai ha−1, respectively) consistently reduced B. tectorum density and cover to very low levels (≤4.8 m−2 and ≤1.3%, respectively) at 45 mo after treatment (MAT), and only modest improvements in control were observed at 57 MAT with two treatments at these rates. Perennial grass cover responded positively to some treatments early in the study (≤33 MAT), but effects were inconsistent across years. Our results suggest that long-term B. tectorum control is possible with a single indaziflam treatment when applying herbicide to small plots, but managers should avoid assuming this outcome will be typical when applying indaziflam at larger scales. The intermediate indaziflam rate evaluated in our study aligns with the maximum single-use application rate permitted by the current grazing label (Rejuvra®, Bayer; 73 g ai ha−1), suggesting that indaziflam may be a powerful tool for land managers tasked with mitigating the impacts of B. tectorum in grazed areas.
Introduction
Downy brome (Bromus tectorum L.) invasion into the sagebrush-grasslands of western North America is one of the most critical threats facing these important rangelands (Clark Reference Clark2020; DiTomaso et al. Reference DiTomaso, Masters and Peterson2010; Smith et al. Reference Smith, Allred, Boyd, Davies, Jones, Kleinhesselink, Maestas, Morford and Naugle2021). Variable germination timing (Beck Reference Beck2009; Knapp Reference Knapp1996; Mack Reference Mack1981), rapid growth (Arredondo et al. Reference Arredondo, Jones and Johnson1998), high seed production (Young et al. Reference Young, Evans, Eckert and Burgess1987), and acquisitive root morphology (Aguirre and Johnson Reference Aguirre and Johnson1991; Arredondo and Johnson Reference Arredondo and Johnson1999), help B. tectorum exploit important soil resources sooner than native plants and promote successful B. tectorum establishment. The altered fire regimes that typically follow invasion favor its increasing dominance, continued spread, and severe impacts to native plant communities (Clark Reference Clark2020; Davies Reference Davies2011; Davies et al. Reference Davies, Leger, Boyd and Hallett2021b; West Reference West and West1983).
Historical fire regimes are difficult to determine in relatively arid plant communities with few trees, but the long recovery time of big sagebrush (Artemisia tridentata Nutt.) suggests that fires were relatively infrequent in the past and limited in their extent (Miller et al. Reference Miller, Chambers, Pyke, Pierson and Williams2013; Schlaepfer et al. Reference Schlaepfer, Lauenroth and Bradford2014). This fire regime is thought to have maintained a mosaic of shrub- and perennial grass–dominated plant communities in different phases of recovery from wildfire (Davies and Bates Reference Davies and Bates2020; McAdoo et al. Reference McAdoo, Schultz and Swanson2013), and supported a variety of different habitat types for the region’s diverse wildlife (Burkhardt Reference Burkhardt1996; McAdoo et al. Reference McAdoo, Swanson, Schultz and Brussard2004). After invasion, more and more continuous fine fuel resulting from B. tectorum litter can substantially increase the likelihood of wildfire ignition and the rate of wildfire spread where and when B. tectorum occurs (Balch et al. Reference Balch, Bradley, D’Antonio and Gómez-Dans2013; Bradley et al. Reference Bradley, Curtis, Fusco, Abatzoglou, Balch, Dadashi and Tuanmu2018; Davies and Nafus Reference Davies and Nafus2013). This is particularly the case when fine fuel accumulates over multiple years with above average precipitation (Pilliod et al. Reference Pilliod, Welty and Arkle2017).
Frequent wildfires can be difficult for relatively slow-growing native perennials to cope with, but annual B. tectorum can recover rapidly (Humphrey and Schupp Reference Humphrey and Schupp2001; Perryman et al. Reference Perryman, Schultz, Burrows, Shenkoru and Wilker2020; Young and Evans Reference Young and Evans1978; Young et al. Reference Young, Evans, Eckert and Burgess1987), resulting in a destructive grass–wildfire feedback loop similar to what has emerged after nonnative grass invasion in many different arid and semiarid ecosystems (D’Antonio and Vitousek Reference D’Antonio and Vitousek1992; Fusco et al. Reference Fusco, Finn, Balch, Nagy and Bradley2019). Across 51 paired burned and unburned sagebrush sites, Swanson et al. (Reference Swanson, Murphy, Swanson, Schultz and McAdoo2018) found that native plant dominance declined after fire in all cases where pre-fire B. tectorum cover exceeded 15%, and Bradley et al. (Reference Bradley, Curtis, Fusco, Abatzoglou, Balch, Dadashi and Tuanmu2018) estimated that B. tectorum cover exceeds 15% over approximately 30% of the U.S. Intermountain West. Estimates suggest that the current extent of western North America’s sagebrush steppe represents roughly half of what it was historically (Miller et al. Reference Miller, Knick, Pyke, Meinke, Hanser, Wisdom, Hild, Knick and Connelly2011), in large part due to this destructive fire–invasion feedback loop.
The rate of B. tectorum expansion further clarifies the need for new tools and innovative approaches to annual grass management. Smith et al. (Reference Smith, Allred, Boyd, Davies, Jones, Kleinhesselink, Maestas, Morford and Naugle2021) estimated that the area of B. tectorum–dominated communities has increased 8-fold since 1990 in the Great Basin, with the most rapid expansion occurring in the most recent decade they considered (2011 to 2020). While relatively cold, high-elevation rangelands have long been considered more resistant to B. tectorum invasion (Chambers et al. Reference Chambers, Bradley, Brown, D’Antonio, Germino, Grace, Hardegree, Miller and Pyke2014), this may be changing, and expansion into higher-elevation rangelands would allow B. tectorum invasion to continue unabated over an even larger portion of the region (Mealor et al. Reference Mealor, Cox and Booth2012; Smith et al. Reference Smith, Allred, Boyd, Davies, Jones, Kleinhesselink, Maestas, Morford and Naugle2021). Unless effective management interventions are developed and deployed, B. tectorum is likely to continue severely impacting rangeland ecosystems in western North America, incurring substantial costs associated with wildland firefighting and restoring repeatedly burned landscapes (Davies et al. Reference Davies, Leger, Boyd and Hallett2021b; Mack Reference Mack and Richardson2011; Perryman et al. Reference Perryman, Schultz, McAdoo, Alverts, Cervantes, Foster, McCuin and Swanson2018).
Existing approaches to manage B. tectorum often provide short-term reductions in abundance (≤2 yr after treatment [YAT]), but long-term control is difficult to achieve, and reinvasion is common without continued management (Mack 2011; Monaco et al. Reference Monaco, Mangold, Mealor, Mealor and Brown2017). Imazapic is a broad-spectrum herbicide that inhibits the enzyme acetolactate synthase; it is selective against annual grasses at low use rates and is likely the most widely used herbicide for managing annual grasses because of its ability to provide both pre- and postemergent control (Kyser et al. Reference Kyser, Wilson, Zhang and Ditomaso2013; Mangold et al. Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013). Imazapic has provided variable results (Applestein et al. Reference Applestein, Germino and Fisk2018; Mangold et al. Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013), often reducing B. tectorum in the first year after treatment but having inconsistent long-term effects on B. tectorum abundance (Davison and Smith Reference Davison and Smith2007; Elseroad and Rudd Reference Elseroad and Rudd2011; Morris et al. Reference Morris, Monaco and Rigby2009; Munson et al. Reference Munson, Long, Decker, Johnson, Walsh and Miller2015).
Indaziflam, a recently labeled herbicide for use on rangelands grazed by livestock (USEPA 2020), provides multiyear B. tectorum control (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). Indaziflam is a cellulose biosynthesis inhibitor with a unique site of action and no reported cases of resistance (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and DeBolt2014; Tateno et al. Reference Tateno, Brabham and Debolt2016). This herbicide is uniquely suited to managing B. tectorum because of its selectivity and long period of residual activity (Clark Reference Clark2020; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016). Indaziflam binds tightly to soil organic matter and remains near the soil surface, where it can selectively inhibit root growth of germinating B. tectorum seeds without harming established perennials with deeper roots (Clark Reference Clark2020; Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). Further, because it typically provides 3 or more years of control, and B. tectorum seedbanks are generally short-lived in the field (≤5 yr; Burnside et al. Reference Burnside, Wilson, Weisberg and Hubbard1996; Sebastian et al. Reference Sebastian, Nissen, Sebastian and Beck2017b; Smith et al. Reference Smith, Meyer and Anderson2008), indaziflam could deplete B. tectorum seedbanks with a single application or a sequence of applications spaced several years apart. While non-target impacts to native annual plants have been observed (Courkamp et al. Reference Courkamp, Meiman and Paschke2022), field trials across the western United States have demonstrated indaziflam’s effectiveness for controlling invasive annual grasses with no apparent impacts to established perennial plants (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019, Reference Clark, Sebastian, Nissen and Sebastian2020; Hart and Mealor Reference Hart and Mealor2021; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a).
Perennial bunchgrasses are a key component of rangeland plant communities that increase resistance and resilience to annual grass invasion and wildfire, respectively (Applestein and Germino Reference Applestein and Germino2022; Blank et al. Reference Blank, Clements, Morgan, Harmon and Allen2020; Chambers et al. Reference Chambers, Bradley, Brown, D’Antonio, Germino, Grace, Hardegree, Miller and Pyke2014; Davies and Johnson Reference Davies and Johnson2017). Thus, proactively treating invaded areas that continue to support relatively abundant perennial grasses may represent a highly effective approach to B. tectorum management. While sagebrush-associated perennial grasses typically live longer than the expected period of residual activity from indaziflam treatment (Svejcar et al. Reference Svejcar, James, Hardegree and Sheley2014), they rely on recruitment from seed for long-term persistence (Hamerlynck and Davies Reference Hamerlynck and Davies2019), which suggests that they may be sensitive to non-target impacts from repeated indaziflam treatments that extend residual activity over longer periods of time.
The primary objective of our study was to evaluate the long-term (57 mo) effectiveness of indaziflam (51, 73, and 102 g ai ha−1) and imazapic (123 g ai ha−1) for controlling B. tectorum in high-elevation sagebrush-grasslands where invaded communities continue to support abundant perennials. We also assessed treatment effects on co-occurring perennial grasses over this same span of time to evaluate the potential for non-target impacts and compared one and two applications of each herbicide treatment (45 mo between applications) to make the study more relevant to land managers considering multiple applications.
Materials and Methods
Site Description
The experiments were established in 2016 at two sites in Sublette County, WY. Site 1 (42.855°N, 109.655°W, approx. 2,250-m elevation) was located near Boulder Lake in the Bridger-Teton National Forest, and Site 2 (42.885°N, 109.739°W, approx. 2,250-m elevation) was located in the Half Moon Habitat Management Unit managed by the Wyoming Game and Fish Department. These study sites were approximately 8 km apart in the Cold Desert region of the North American Deserts ecoregion; Site 1 was characterized as a coarse upland ecological site (R043BY208WY), and Site 2 was characterized as a shallow loamy ecological site (R043BY162WY; USDA-NRCS 2021).
The soil at Site 1 was Pointer-Lateral complex (loamy-skeletal, mixed, superactive, Ustic Haplocryolls), which is characterized by a very cobbly sandy loam surface soil with 2.9% organic matter and 6.8 pH in the top 20 cm (USDA-NRCS 2021). The soil at Site 2 was Blackbear, rubbly-Branham, rubbly-Bobowic complex (loamy-skeletal, mixed, superactive, Pachic Agricryolls), which is characterized by cobbly or gravelly coarse sandy loam surface soil with 5.3% organic matter and 6.6 pH in the top 20 cm (USDA-NRCS 2021). Both sites had south-facing aspects, but Site 1 was slightly steeper than Site 2.
When treatments were applied, both sites supported plant communities dominated by native perennial bunchgrasses and shrubs, but invaded such that the majority of interspaces between established plants were infested by B. tectorum. The most common perennial grasses at both sites were needle and thread [Hesperostipa comata (Trin. & Rupr.) Barkworth] and bluebunch wheatgrass [Pseudoroegneria spicata (Pursh) Á. Löve], and the most common shrubs included antelope bitterbrush [Purshia tridentata (Pursh) DC], mountain big sagebrush [Artemisia tridentata Nutt. ssp. vaseyana (Rydb.) Beetle], and mountain snowberry [Symphoricarpos oreophilus A. Gray]. The dominant forb at both sites was arrowleaf balsamroot [Balsamorhiza sagittata (Pursh) Nutt.]. A variety of less common native and nonnative plants existed at low abundance at the time of treatment.
Mean annual precipitation based on the 30-yr mean (1981 to 2010) was 294 mm for both sites based on the nearest weather station (located in Pinedale, 16 km from Site 1, 9 km from Site 2; WRCC 2021). Relative to this average, the 2017 and 2019 water years (October to September) were particularly wet (481 mm and 421 mm, respectively), and 2021 was particularly dry (250 mm). All other study years (2016, 2018, 2020) were within 10% of the 30-year mean (WRCC 2021).
An incidental, human-ignited wildfire (Boulder Lake Fire) burned Site 1 in August 2019. The fire was ignited on August 17, 100% contained on August 26, and declared out on September 17 (Teton Interagency Fire 2019). All study plots at this site were completely burned, and data from 45 and 57 mo after treatment (MAT) at Site 1 were collected approximately 9 and 21 mo after the fire, respectively.
Experimental Design
Initial herbicide treatments were applied at both sites on September 9, 2016, using a CO2-pressurized custom-built backpack sprayer with 11002LP flat-fan nozzles (TeeJet® Spraying Systems, P.O. Box 7900, Wheaton, Il 60187) delivering 187 L ha−1 at 207 kPa. Along with an untreated control, four herbicide treatments were applied to 3 by 9 m plots in a randomized complete block design with four replications at each site. Herbicide treatments included three indaziflam rates (51, 73, and 102 g ha−1; henceforth low, intermediate, and high rates, respectively) and imazapic applied at 123 g ha−1. At the time of application, native plants were dormant, B. tectorum was 100% post–seed set, and no fall B. tectorum emergence was observed.
The same herbicide treatments were reapplied to half of each treated plot approximately 45 mo after initial herbicide application to evaluate long-term reductions in B. tectorum abundance with one and two applications of each treatment. This resulted in eight different herbicide treatments (one and two applications of four treatments, 3 by 4.5 m plots) along with an undivided control plot. Reapplication occurred on June 26, 2020, at Site 1 and June 27, 2020, at Site 2 using a CO2-pressurized handheld research sprayer with 8002VS flat-fan nozzles (TeeJet® Spraying Systems) delivering 187 L ha−1 at 207 kPa. At the time of reapplication, native plants were still actively growing, and B. tectorum was nearing 100% post–seed set. All treatments (initial and reapplication) included a 0.25% v/v nonionic surfactant.
Treatment Evaluations and Data Analysis
To quantify herbicide treatment effects, we used 0.5-m2 frames (Bonham et al. Reference Bonham, Mergen and Montoya2004) to measure B. tectorum density and perennial grass and B. tectorum absolute canopy cover (plant canopy relative to ground area; henceforth cover). We counted individual B. tectorum plants and recorded ocular estimates of cover to the nearest 1% in these frames. When B. tectorum was especially dense, plants were counted in only a portion of the larger frame to estimate density. Before herbicide reapplication, data were collected from five randomly located frames (subsamples) in each plot, and after reapplication, data were collected from three randomly located frames in each divided treatment plot. Data were collected at 9, 21, 33, 45, and 57 MAT (June 2017 to 2021), with data collection at 57 MAT occurring at 12 mo after reapplication.
To test treatment effects on B. tectorum density and B. tectorum and perennial grass cover before reapplication, we used the lme4 package in R. v. 3.6.2 (R Core Team 2019) to create linear mixed-effects models and ANOVA to test for treatment effects at α = 0.05. Due to substantial interannual variability and environmental differences between sites, site and year were analyzed independently in all cases, with block included as a random factor. Visual inspection of quantile-quantile and fitted versus residual plots was used to verify that data met the assumptions of ANOVA. Cover data were arcsine square-root transformed, and density data were square-root transformed (n + 0.5) as necessary to meet these assumptions. When ANOVA indicated that significant differences existed between treatments, we used the emmeans package (R Core Team 2019) to obtain pairwise comparisons between treatment groups using a Tukey adjustment (α = 0.05).
After reapplication, our experiment did not represent a true split-plot experimental design, because no sequential treatment was applied to the control plots, and these plots remained undivided. Thus, to analyze data from 57 MAT, each herbicide treatment and sequence (one or two applications) was considered a single experimental treatment; data included eight herbicide treatments and an untreated control at 57 MAT. Because B. tectorum was nearly absent from all plots at Site 2 at this time, including nontreated controls, we only compared B. tectorum cover and density at 57 MAT at Site 1. Otherwise, data were analyzed using the same procedure used to compare treatments before reapplication. Results are presented in their original dimensions, means are reported with standard errors, and P-values reported in the text result from post hoc mean comparisons of treatments and untreated controls (Tukey’s honest significant difference).
Results and Discussion
Bromus tectorum Density and Cover
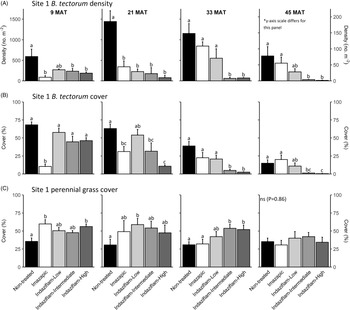
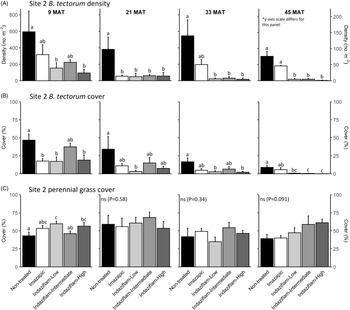
Table 1 shows ANOVA results for B. tectorum density and cover. At Site 1, all treatments reduced B. tectorum density at 9 MAT, except the low indaziflam rate (imazapic P < 0.001; low P = 0.11; intermediate P = 0.041; high P = 0.017; Figure 1A), and all treatments reduced B. tectorum density at 21 MAT (P < 0.01; Figure 1A). At Site 2, responses to treatments at 9 MAT were more variable; B. tectorum density was only reduced compared with the nontreated control in plots treated with the low and high indaziflam rates (low P = 0.020; high P < 0.01; Figure 2A). However, similar to Site 1, all treatments reduced B. tectorum density at 21 MAT (imazapic P = 0.027; low P < 0.01; intermediate P = 0.032; high P = 0.014; Figure 2A). The more variable results at Site 2 may be the result of unexpected fall B. tectorum emergence occurring before herbicide application in September 2016. Indaziflam has no postemergence activity, so B. tectorum plants that emerged before application would not be controlled the following spring. While no fall emergence was detected at the time of treatment, dense B. tectorum litter was present at both sites, and this may have made emergence difficult to observe.
Table 1. Results of ANOVA (α = 0.05) for treatment effects on Bromus tectorum density, B. tectorum cover, and perennial grass cover at Site 1 (n = 4) and Site 2 (n = 4).
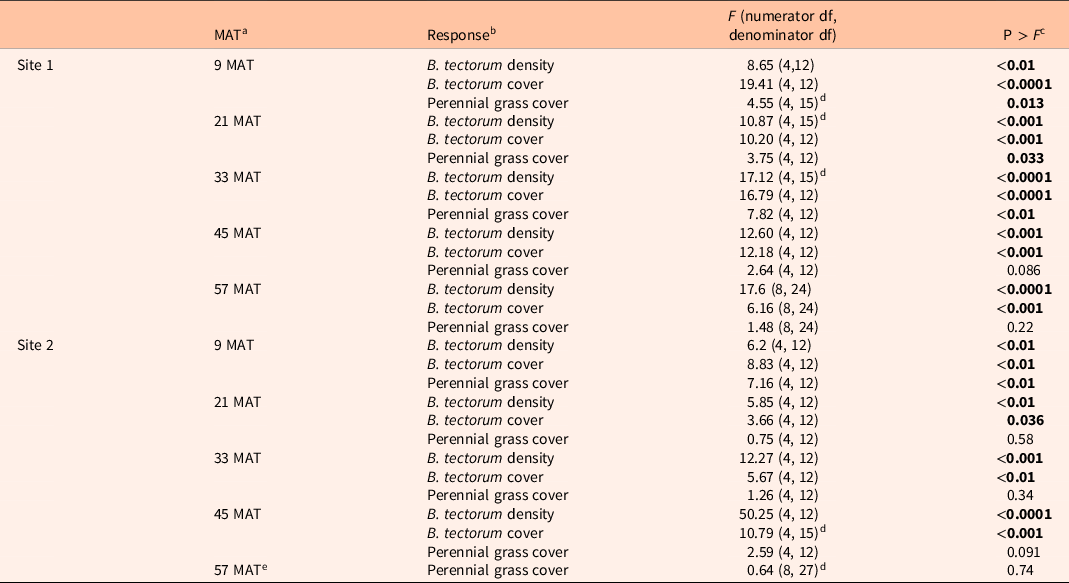
a MAT, months after treatment.
b Density = number of individuals m−2; cover = absolute canopy cover.
c Significant effects (P < 0.05) shown in bold.
d Random effect (block) was estimated as zero and removed from the model.
e Treatment effects on B. tectorum density and cover were not assessed at 57 MAT at Site 2 due to the near complete absence of B. tectorum.
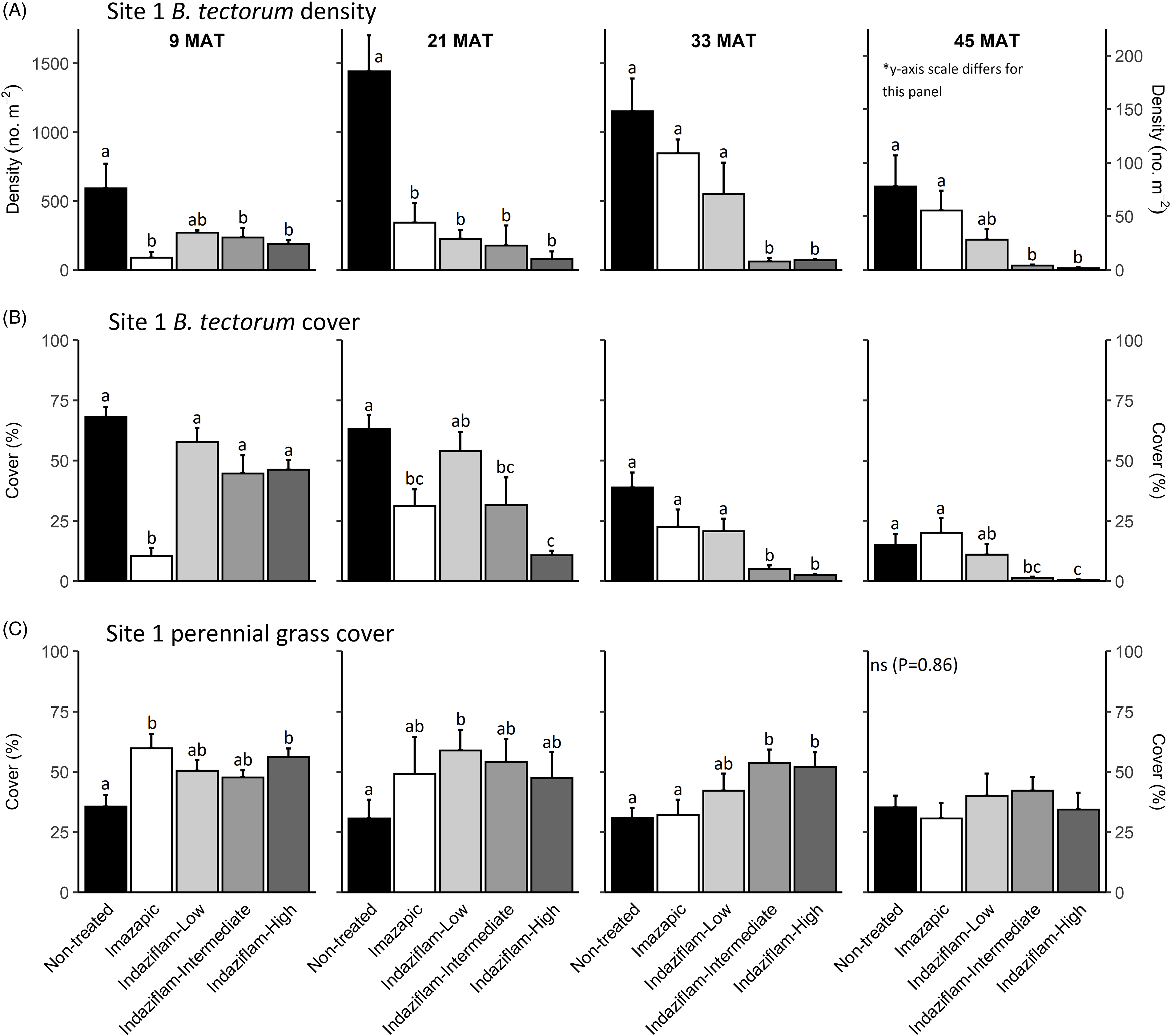
Figure 1. Mean (+1 SE) Bromus tectorum density (A), B. tectorum cover (B), and perennial grass cover (C) at Site 1 (Boulder Lake) at 9, 21, 33, and 45 mo after treatment (MAT; cover = absolute canopy cover). Herbicide treatments were applied in September 2016 when native plants were dormant and B. tectorum was 100% post–seed set. Letters indicate significant within-year differences among treatment means (Tukey’s honest significant difference, α = 0.05, n = 4). Herbicide treatments are as follows: imazapic = imazapic 123 g ai ha−1; indaziflam-low = indaziflam 44 g ai ha−1; indaziflam-intermediate = indaziflam 73 g ai ha−1; indaziflam-high = indaziflam 102 g ai ha−1. All treatments included a 0.25% v/v nonionic surfactant. Note that y-axis scale is consistent across panel rows in all cases, except for B. tectorum density at 45 MAT (*).
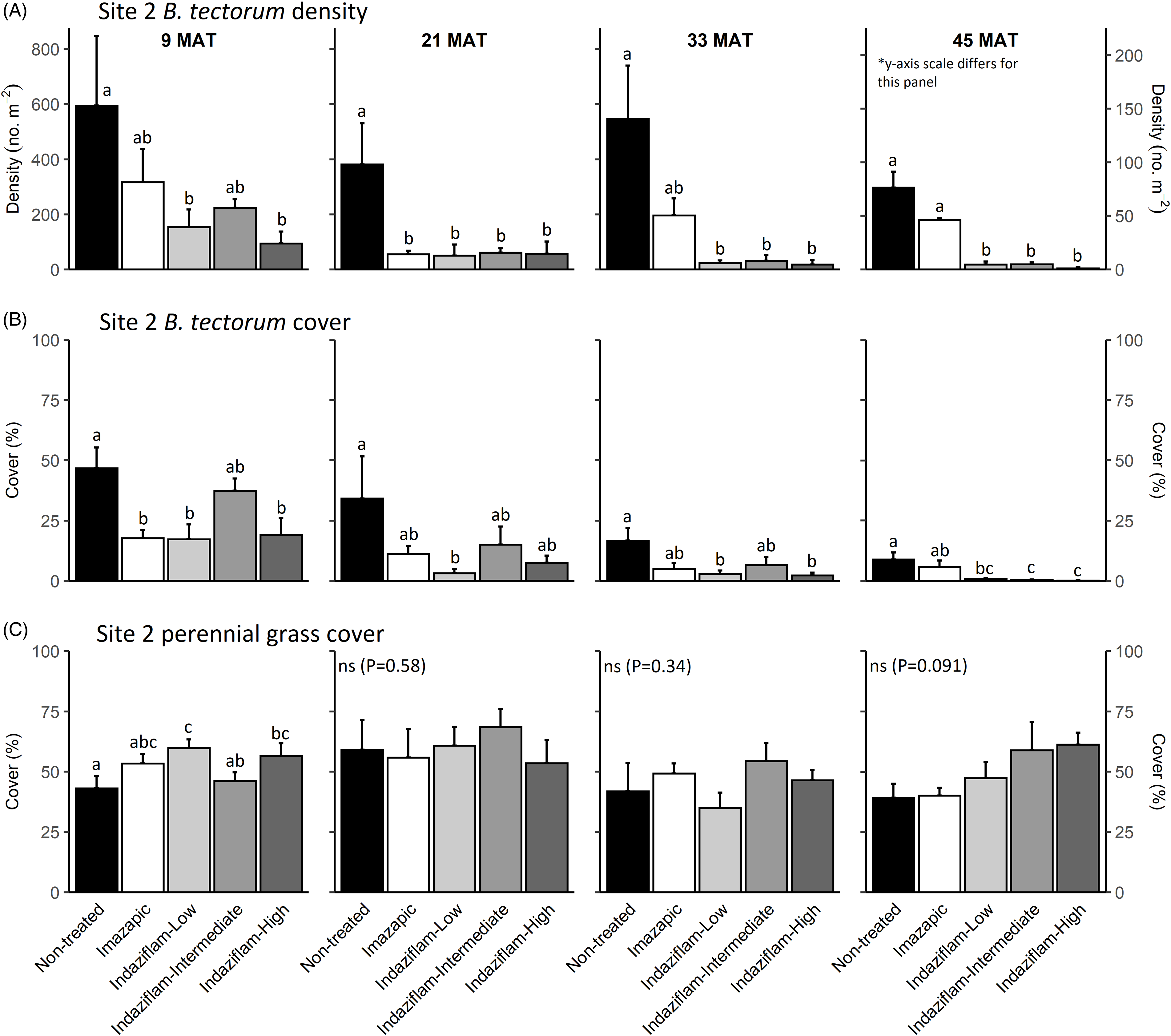
Figure 2. Mean (+1 SE) Bromus tectorum density (A), B. tectorum cover (B), and perennial grass cover (C) at Site 2 (Half Moon) at 9, 21, 33, and 45 mo after treatment (MAT; cover = absolute canopy cover). Herbicide treatments were applied in September 2016 when native plants were dormant and B. tectorum was 100% post–seed set. Letters indicate significant within-year differences among treatment means (Tukey’s honest significant difference, α = 0.05, n = 4). Herbicide treatments are as follows: imazapic = imazapic 123 g ai ha−1; indaziflam-low = indaziflam 44 g ai ha−1; indaziflam-intermediate = indaziflam 73 g ai ha−1; indaziflam-high = indaziflam 102 g ai ha−1. All treatments included a 0.25% v/v nonionic surfactant. Note that y-axis scale is consistent across panel rows in all cases, except for B. tectorum density at 45 MAT (*).
In contrast to the variability observed at 9 and 21 MAT, the intermediate and high indaziflam rates reduced B. tectorum density compared with the nontreated control at both sites at 33 and 45 MAT (P < 0.01; Figures 1A and 2A). The effects of imazapic and the low indaziflam rate were less consistent as the study progressed (Figures 1A and 2A). At Site 1, imazapic and the low indaziflam rate did not reduce B. tectorum density compared with the nontreated control at 33 MAT (imazapic P = 0.82; low P = 0.11) and 45 MAT (imazapic P = 0.85; low P = 0.13; Figure 1A). The effects of imazapic at Site 2 mirrored those of Site 1, with no effects on B. tectorum density at 33 MAT (P = 0.10) and 45 MAT (P = 0.11; Figure 2A). However, reductions in B. tectorum density were comparable for all indaziflam rates at Site 2, with significant reductions observed at 33 and 45 MAT (P < 0.01; Figure 2A).
The effects of treatment on B. tectorum cover were less consistent (Figures 1B and 2B). At Site 1, only the imazapic treatment reduced B. tectorum cover compared with the nontreated control at 9 MAT (P < 0.001); all treatments except the low indaziflam rate (P = 0.66) reduced B. tectorum cover at 21 MAT (imazapic P = 0.030; intermediate P = 0.033; high P < 0.001); and only the intermediate and high indaziflam rates reduced B. tectorum cover at 33 (P < 0.001) and 45 MAT (intermediate P = 0.013; high P < 0.01; Figure 1B). At Site 2, all treatments except the intermediate rate of indaziflam (P = 0.62) reduced B. tectorum cover compared with the nontreated control at 9 MAT (P < 0.01); only the low indaziflam rate reduced B. tectorum cover at 21 MAT (P = 0.023); the low and high indaziflam rates reduced B. tectorum cover at 33 MAT (low P = 0.025; high P = 0.018), and all indaziflam treatments reduced B. tectorum cover at 45 MAT (P < 0.01; Figure 2B).
Variability in treatment effects on B. tectorum cover at 9 and 21 MAT (Figures 1B and 2B) is similar to what we observed for B. tectorum density (Figures 1A and 2A) and may also be explained by unexpected and undetected fall B. tectorum emergence the year of treatment. However, less consistent effects of indaziflam treatment on B. tectorum cover may also be influenced by the environmental conditions that B. tectorum individuals that escape control experience after indaziflam treatment eliminates most intraspecific competitors. Plants in dense monocultures often have only one or a few tillers, while solitary B. tectorum individuals in resource-rich environments often have many tillers and produce thousands of seeds (Hulbert Reference Hulbert1955; Young et al. Reference Young, Evans, Eckert and Burgess1987; Zouhar Reference Zouhar2003). These plants may also have large impacts on cover with minimal effects on density (Elzinga et al. Reference Elzinga, Salzer and Willoughby1998). While mean B. tectorum cover was very low in plots treated with intermediate and high indaziflam rates at both sites at 45 MAT (Figures 1B and 2B), we infrequently observed solitary B. tectorum individuals with 25+ tillers in indaziflam-treated plots, and these large plants may have contributed to the inconsistent treatment effects on B. tectorum cover we observed.
Based on prior research (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016), we expected indaziflam to reduce B. tectorum cover and density for at least 3 yr, particularly at intermediate and high application rates, and imazapic to provide effective short-term control (1 to 2 YAT), but not long-term control (≥2 YAT). Consistent with this expectation, intermediate and high indaziflam rates consistently reduced B. tectorum density and cover to very low levels at 33 and 45 MAT at both sites, and imazapic was generally effective in the near term, but B. tectorum density and cover were not reduced in imazapic-treated plots beyond 21 MAT (Figures 1A and B and 2A and B). Importantly, the intermediate indaziflam rate evaluated in our study aligns with the maximum single-use application rate permitted by the current grazing label (Rejuvra®, Bayer; 73 g ai ha−1; USEPA 2020), suggesting that indaziflam treatment at this rate may be a powerful tool for land managers tasked with mitigating the impacts of B. tectorum in grazed areas.
We observed an overall decline in B. tectorum abundance at our sites during the study. This decline is best reflected by B. tectorum cover, which steadily declined in nontreated control plots at both sites over the course of the study (Figures 1B and 2B). Bromus tectorum density remained high in nontreated control plots through 33 MAT at both sites (592 to 1,441 m−2 at Site 1 and 381 to 594 m−2 at Site 2), but it was much lower at 45 MAT (78 m−2 at Site 1 and 76 m−2 at Site 2; Figures 1A and 2A), and B. tectorum was nearly absent from all plots at 57 MAT at Site 2 (data not shown). This natural decline may reflect dry spring conditions; other research has observed temporary declines in B. tectorum abundance at high elevations related to periodic spring drought (Smith et al. Reference Smith, Allred, Boyd, Davies, Jones, Kleinhesselink, Maestas, Morford and Naugle2021). In the months leading up to sampling at 57 MAT (June 2021), spring precipitation (March to June) in nearby Pinedale, WY, was reduced roughly 30% compared with the 30-yr mean (83 mm vs. 121 mm), with particularly dry conditions in June (4 mm vs. 31 mm; WRCC 2021).
Higher-elevation sagebrush communities are also thought to present greater challenges to B. tectorum invasion due to colder soil temperatures and more abundant perennial grasses (Chambers et al. Reference Chambers, Bradley, Brown, D’Antonio, Germino, Grace, Hardegree, Miller and Pyke2014), and these same challenges could contribute to B. tectorum declines under the right circumstances. Local weed managers have noted declines in B. tectorum following cold spring conditions (J Kraft, personal communication), and one well-studied montane B. tectorum population located at a similar elevation (2,328 m) is known to have disappeared between 2005 and 2012 in Utah (Merrill et al. Reference Merrill, Meyer and Coleman2012). Both our sites are located at the same elevation (approx. 2,250 m), but the decline was more pronounced at Site 2, suggesting that elevation is likely not the only contributing factor. Differences between the sites may be related to differences in the degree of slope; both sites have south-facing aspects, but Site 1 may be slightly warmer due to its steeper slope, and this may favor B. tectorum by allowing it to begin growing earlier in the season, when resources are abundant and competition with native plants is minimal. We cannot completely explain the natural decline in B. tectorum abundance that occurred during our study, but it suggests that relatively high-elevation sagebrush-grasslands may not be highly suitable habitat for B. tectorum every year.
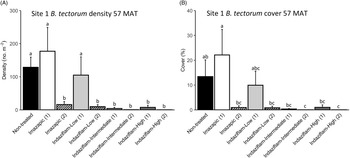
Due to the near absence of B. tectorum at 57 MAT at Site 2, we only analyzed B. tectorum cover and density after reapplication at Site 1 (Table 1). All treatments except single applications of imazapic (P > 0.9) and the low indaziflam rate (P > 0.9) reduced B. tectorum density compared with the nontreated control at 57 MAT (P < 0.001; Figure 3A), and there was no difference in B. tectorum density between treatment groups that received one or two applications of the intermediate and high indaziflam rates (P > 0.9; Figure 3A). Treatment effects on B. tectorum cover after reapplication were similar to those seen for density; the differences between treatment groups that received one or two applications of the intermediate and high rates of indaziflam were nonsignificant (P > 0.9; Figure 3B). However, B. tectorum cover was only reduced compared with the nontreated control at 57 MAT by two indaziflam applications at these same rates (intermediate P = 0.028; high P = 0.027; Figure 3B). Mean B. tectorum cover at 57 MAT was still very low in plots that received only one application of the intermediate and high rates of indaziflam (Figure 3B), but differences between these plots and the nontreated controls were only significant at the α = 0.10 level (intermediate P = 0.071; high P = 0.092).
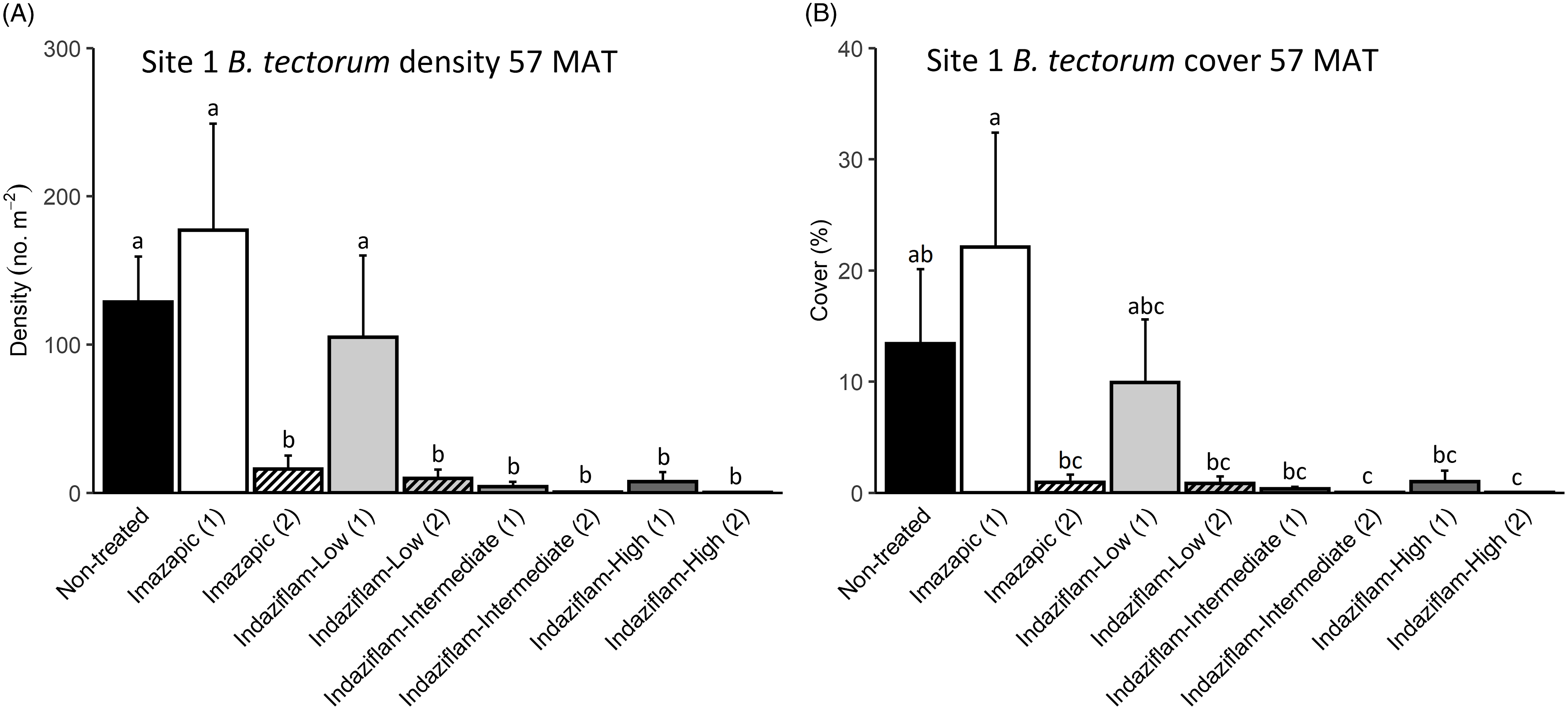
Figure 3. Mean (+1 SE) Bromus tectorum density (A) and B. tectorum cover (B) at Site 1 (Boulder Lake) at 57 mo after treatment (MAT; cover = absolute canopy cover). Treatment groups followed by a (1) received only one herbicide application, and treatment groups followed by a (2) received a sequence of two herbicide applications (diagonal line pattern). Initial herbicide treatments were applied in September 2016, and reapplications of the same treatments were made approximately 45 mo later in June 2020. Native plants were dormant and B. tectorum was 100% post–seed set when initial treatments were applied, and native plants were actively growing and B. tectorum was near 100% post–seed set when reapplication occurred. Letters indicate significant differences among treatment means (Tukey’s honest significant difference, α = 0.05, n = 4). Herbicide treatments are as follows: imazapic = imazapic 123 g ai ha−1; indaziflam-low = indaziflam 44 g ai ha−1; indaziflam-intermediate = indaziflam 73 g ai ha−1; indaziflam-high = indaziflam 102 g ai ha−1. All treatments (initial and reapplication) included a 0.25% v/v nonionic surfactant.
We expected reapplication to be necessary to maintain reductions in B. tectorum density and cover 3+ yr after initial treatment. This expectation was based on previous research showing that B. tectorum seedbanks can last 4 to 5 yr (Sebastian et al. Reference Sebastian, Nissen, Sebastian and Beck2017b; Young et al. Reference Young, Evans, Eckert and Burgess1987), and a companion study near Site 1 that detected only trace amounts of indaziflam in the soil at 37 MAT in plots treated aerially with the intermediate indaziflam rate (Courkamp et al. Reference Courkamp, Meiman and Paschke2022). However, we also acknowledged that it may be possible to deplete B. tectorum seedbanks more quickly in some environments, because a variety of factors, including climate, fire history, and the specifics of the soil resource environment, can affect the likelihood of seed germination and seedbank longevity in the field (Baskin and Baskin Reference Baskin and Baskin2014; Bazzaz Reference Bazzaz1996; Evans and Young Reference Evans and Young1975). One and two applications of the intermediate and high indaziflam rates resulted in comparable reductions in B. tectorum density and cover at 57 MAT at Site 1 (Figure 3A and B), suggesting that it may be possible to deplete B. tectorum seedbanks and achieve long-term control with only one herbicide application.
It is unlikely that indaziflam is still actively inhibiting B. tectorum at 45 and 57 MAT at our site (Courkamp et al. Reference Courkamp, Meiman and Paschke2022), but if near-complete seedbank depletion occurs in the years immediately following application, control may continue until B. tectorum seeds disperse into treated areas from elsewhere. Further, relatively more precocious seed germination has been associated with B. tectorum populations collected in higher-elevation montane environments similar to our study sites (Allen and Meyer Reference Allen and Meyer2002; Meyer and Allen Reference Meyer and Allen1999), and this may promote more rapid seedbank depletion, because a greater fraction of the B. tectorum seeds present at the time of treatment are likely to germinate in the period of residual activity following a single application. Many factors can influence both B. tectorum seed longevity in field conditions (Baskin and Baskin Reference Baskin and Baskin2014; Bazzaz Reference Bazzaz1996; Evans and Young Reference Evans and Young1975) and the likelihood of seed dispersal (e.g., Monty et al. Reference Monty, Brown and Johnston2013), thus we caution against assuming this outcome will be typical across different circumstances.
Our results demonstrate the potential of indaziflam for managing invasive annual grasses, but land managers should consider that our treatments were applied using a research sprayer that ensures consistent herbicide coverage in small plots. Even with this equipment, none of our treatments completely eliminated B. tectorum, and indaziflam is likely to be applied aerially and at much larger scales than our experimental plots. A companion study located near Site 1 (<200 m between study sites) evaluated aerial applications (helicopter) of the intermediate indaziflam rate to 2-ha plots (73 g ai ha−1 indaziflam with 47 L ha−1 of water as the carrier). Reductions in B. tectorum density and cover were more variable in these circumstances (Courkamp et al. Reference Courkamp, Meiman and Paschke2022), suggesting that the risk of forgoing a second treatment and allowing B. tectorum to reestablish is worth considering. Bromus tectorum is known for rapid population growth (Humphrey and Schupp Reference Humphrey and Schupp2001; Perryman et al. Reference Perryman, Schultz, Burrows, Shenkoru and Wilker2020; Young and Evans Reference Young and Evans1978), and if less consistent herbicide coverage allows B. tectorum persistence in treated areas, it may rapidly replenish the seedbank after residual activity wanes and force land managers to start the process of depleting the seedbank for a second time. A sequence of two treatments may reduce this risk by increasing the consistency of treatment outcomes and the likelihood of achieving near-complete seedbank depletion at meaningful spatial scales, but future research is necessary to evaluate single and multiple indaziflam applications in operational settings. Land managers who use indaziflam in the near term will have to weigh the potential risk of reinvasion against the cost of indaziflam and make the best possible decision in each case given the resources available for treatment.
Perennial Grass Cover
We expected indaziflam treatments to have no observable impact on the cover of co-occurring perennial grasses based on previous research (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020; Hart and Mealor Reference Hart and Mealor2021; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). Consistent with our expectation, we did not observe any negative effects on perennial grass cover (Figures 1C and 2C). However, we observed positive effects on perennial grass cover early in the study, with perennial grass cover increasing relative to the nontreated control at 9 MAT in plots treated with imazapic and the high indaziflam rate at Site 1 (imazapic P = 0.014; high P = 0.039; Figure 1C), and plots treated with the low and high indaziflam rates at Site 2 (low P < 0.01; high P = 0.024; Figure 2C). Beyond 9 MAT, we observed no treatment effects on perennial grass cover at Site 2 (P > 0.09; Figure 2C), including at 57 MAT in plots where herbicide treatments were reapplied (19.8% to 32.0% mean perennial grass cover; data not shown). At Site 1, perennial grass cover was higher than in the nontreated control at 21 MAT in plots treated with the low indaziflam rate (P = 0.026), and the same was true at 33 MAT in plots treated with the intermediate and high indaziflam rates (intermediate P < 0.01; high P = 0.015; Figure 1C). Beyond 33 MAT, we observed no treatment effects on perennial grass cover at Site 1 (P > 0.22; Figure 1C), including at 57 MAT in plots where herbicide treatments were reapplied (27.0% to 40.9% mean perennial grass cover; data not shown).
Positive effects on perennial grass biomass following indaziflam treatment have been observed in some studies (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020; Hart and Mealor Reference Hart and Mealor2021; Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a), and resource preemption by B. tectorum is relatively well understood and can have substantial negative effects on perennial grass growth and vigor (Melgoza et al. Reference Melgoza, Nowak and Tausch1990; Nasri and Doescher Reference Nasri and Doescher1995; Ploughe et al. Reference Ploughe, Carlyle and Fraser2020). Consistent with this explanation, perennial grass cover at 33 MAT at Site 1 showed a positive response at intermediate and high indaziflam rates (Figure 1C), where B. tectorum density and cover were also lowest at this time (Figure 1A and B). These data support previous studies demonstrating that the positive perennial grass cover responses we observed probably resulted from reduced B. tectorum competition (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2020; Hart and Mealor Reference Hart and Mealor2021; Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a).
If positive perennial grass cover responses were the result of reduced annual grass competition, it is not surprising that differences between treatments diminished over time at Site 2, where the natural decline in B. tectorum abundance that occurred during our study was most pronounced. At Site 1, the wildfire that occurred in August 2019 likely precluded our ability to detect treatment effects on perennial grass cover at 45 and 57 MAT. Our findings add to a growing number of studies demonstrating that indaziflam can selectively control annual grasses with minimal risk to established perennial plants (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019, Reference Clark, Sebastian, Nissen and Sebastian2020; Fowers and Mealor Reference Fowers and Mealor2020; Hart and Mealor Reference Hart and Mealor2021; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a; Seedorf et al. Reference Seedorf, Clark and Nissen2022), but future research should evaluate the potential for longer-term impacts to native perennials with repeated treatments. Impacts to P. spicata and A. tridentata seedlings have been observed in a grow room study (Clenet et al. Reference Clenet, Davies, Johnson and Kerby2019), and grazing managers have long understood the importance of allowing perennial grasses to complete their reproductive cycles in at least some years (Burkhardt and Sanders Reference Burkhardt and Sanders2012), which suggests that the potential for longer-term impacts from repeated indaziflam treatments may be different.
We know of no published studies documenting annual grass reductions resulting from indaziflam treatment over a comparably long period of time (57 MAT), and our results reflect the variability that managers can expect to face when treating B. tectorum in the notoriously heterogeneous and unpredictable rangeland ecosystems of western North America (Boyd and Svejcar Reference Boyd and Svejcar2009; Svejcar et al. Reference Svejcar, Boyd, Davies, Hamerlynck and Svejcar2017). Land managers should be aware that short-term treatment outcomes may be inconsistent, and control may improve over time when using indaziflam alone, as was observed in our study. Seedorf et al. (Reference Seedorf, Clark and Nissen2022) observed more consistent short-term (1 to 2 YAT) B. tectorum control when tank mixing indaziflam with imazapic compared with applying indaziflam on its own, and imazapic consistently reduced B. tectorum abundance at 9 MAT in our study (Figures 1A and B and 2A and B). Coupled with its selectivity against annual grasses at low use rates (Kyser et al. Reference Kyser, Wilson, Zhang and Ditomaso2013), the short-term effectiveness of imazapic may make it a suitable tank-mix partner for indaziflam that can provide reliable short-term B. tectorum control.
Developing and implementing tools with the capacity to effectively manage annual grasses is critical to prevent the annual grass-fueled “downward spiral” of which researchers have long been aware (West Reference West and West1983; Young and Evans Reference Young and Evans1978), and recent research suggesting that B. tectorum may be able to expand its dominance in higher-elevation sagebrush-grasslands only makes the necessity of confronting B. tectorum invasion more salient (Mealor et al. Reference Mealor, Cox and Booth2012; Smith et al. Reference Smith, Allred, Boyd, Davies, Jones, Kleinhesselink, Maestas, Morford and Naugle2021). Expanded dominance in higher-elevation areas also suggests that the unexplained decline in B. tectorum abundance highlighted by our study is likely best understood as a temporary reduction in the density and cover of adult plants and not a durable transition back to a less-invaded state (Davies et al. Reference Davies, Leger, Boyd and Hallett2021b; Smith et al. Reference Smith, Allred, Boyd, Davies, Jones, Kleinhesselink, Maestas, Morford and Naugle2021). Proactive land managers seeking to preserve some of the resistance to invasion and resilience to wildfire conferred by perennial grasses may be able to use indaziflam to reduce B. tectorum where and when it co-occurs with these important rangeland plants, and this may represent a highly effective annual grass management strategy in sagebrush-grasslands.
While the intermediate and high indaziflam rates reduced density and cover to very low levels (Figures 1A and B and 2A and B), no treatment completely eliminated B. tectorum, which is notorious for its ability to rapidly recover from disturbance (Humphrey and Schupp Reference Humphrey and Schupp2001; Perryman et al. Reference Perryman, Schultz, Burrows, Shenkoru and Wilker2020; Young and Evans Reference Young and Evans1978; Young et al. Reference Young, Evans, Eckert and Burgess1987). The scale of B. tectorum invasion and the reality of limited resources for management also make it clear that B. tectorum needs to be managed as a permanent component of rangeland plant communities in western North America (Davies et al. Reference Davies, Leger, Boyd and Hallett2021b; Perryman et al. Reference Perryman, Schultz, McAdoo, Alverts, Cervantes, Foster, McCuin and Swanson2018). In light of these challenges, future research should consider how best to prevent or delay B. tectorum reinvasion in treated areas and reduce the need for additional treatments after initial seedbank depletion (e.g., Davies and Sheley Reference Davies and Sheley2007). Researchers and land managers should also work together to determine how to best deploy indaziflam at landscape scales and combine indaziflam treatment with other practices (e.g., grazing to reduce fine fuels and safe sites for B. tectorum germination; Davies et al. Reference Davies, Boyd, Bates and Hulet2016, Reference Davies, Bates, Perryman and Arispe2021a; Perryman et al. Reference Perryman, Schultz, Burrows, Shenkoru and Wilker2020) and emerging restoration technologies (e.g., seed coatings and herbicide protection pods; Clenet et al. Reference Clenet, Davies, Johnson and Kerby2019; Holfus et al. Reference Holfus, Rios, Boyd and Mata-González2021; Svejcar et al. Reference Svejcar, Brown, Ritchie, Davies and Svejcar2022). Along with other studies (Clark et al. Reference Clark, Sebastian, Nissen and Sebastian2019, Reference Clark, Sebastian, Nissen and Sebastian2020; Hart and Mealor Reference Hart and Mealor2021; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a; Seedorf et al. Reference Seedorf, Clark and Nissen2022), our findings suggest that indaziflam may allow land managers to achieve objectives that were not feasible with other management tools and that indaziflam can play a significant role in efforts to mitigate the devastating impacts of annual grass invasion.
Acknowledgments
The authors would like to thank the Bridger-Teton National Forest (BTNF) for providing the funding for this project (grant nos. 16-CS-11040300-057 and 19-CS-11040300-064), and we would like to specifically thank Dave Cottle (BTNF) and Julie Kraft (Sublette County Weed and Pest) for their support. We would also like to thank Bayer for donating the herbicide used in this research, and Sublette County Weed and Pest for covering application costs. We thank Sublette County Weed and Pest, the BTNF, the Wyoming Game and Fish Department, and the U.S. Natural Resource Conservation Service for the countless staff hours they dedicated to the success of this project. Additionally, Alex Stoneburner, Derek Sebastian, Shannon Clark, Roman Borys, Hadley Manning, and Lars Anderson all provided valuable assistance at various stages during project development, data collection, and manuscript preparation. We would also like to thank two anonymous reviewers for their thoughtful feedback on the initial version of the article. This work was supported by the USDA National Institute of Food and Agriculture, Hatch Project 1012851. No conflicts of interest have been declared.