Management Implications
Benthic matting and hand pulling are frequently proposed to environmental managers as alternatives to herbicides for controlling submerged aquatic plants, in spite of the dearth of scientific research regarding their effectiveness and cost over multiple years. An experiment conducted in a small (≈1 km2) lake invaded by Myriophyllum spicatum (Eurasian watermilfoil) over a 5-yr period showed that it is possible to (1) reduce plant cover by >95% with these physical methods and (2) promote the spontaneous restoration of native plant populations. Control nevertheless requires careful planning, a substantial initial investment in benthic mats, and an annual hand-pulling effort that must be indefinitely maintained to prevent reinfestation. The use of 1,000 m2 of benthic mats requires about 50 person-hours per summer season, including installation, removal, and maintenance. Intensive management (years 1 to 5) using benthic mats and hand pulling cost an estimated Can$185,000 (US$140,000) ha−1 of aquatic plant bed. Effective control of submerged aquatic plants with physical methods is expensive, albeit feasible, even with the contribution of volunteers and the support of various levels of government. The high cost deserves serious consideration; for example, the same money could be used to address more pressing lacustrine environmental issues.
Introduction
Eurasian watermilfoil (Myriophyllum spicatum L., Haloragaceae) is an aquatic vascular plant introduced in North America in the 1940s accidentally or as an aquarium plant. This perennial establishes in freshwater lakes and rivers, generally at depths of 1 to 4 m, where it forms extensive dense beds (Hussner et al. Reference Hussner, Stiers, Verhofstad, Bakker, Grutters, Haury, van Valkenburg, Brundu, Newman, Clayton, Anderson and Hofstra2017; Smith and Barko Reference Smith and Barko1990). The success of M. spicatum in North America may be explained by its rapid growth and low nitrogen and phosphorus requirements. Although M. spicatum can reproduce sexually, new sites are mainly colonized by stem fragments that detach from the mother plants, from mid-July to early October (Aiken et al. Reference Aiken, Newroth and Wiles1979; Grace and Wetzel Reference Grace and Wetzel1978; Madsen et al. Reference Madsen, Eichler and Boylen1988). Fragments that settle on the lake bottom can take root and rapidly initiate new beds (Heidbüchel and Hussner Reference Heidbüchel and Hussner2019; Madsen and Smith Reference Madsen and Smith1997). Humans also play a role in the inter-lake dispersal of M. spicatum by transporting stem fragments on their watercraft or trailers (Boylen et al. Reference Boylen, Eichler, Bartkowski and Shaver2006; Bruckerhoff et al. Reference Bruckerhoff, Havel and Knight2014; Kao et al. Reference Kao, Enns, Tomamichel, Doll, Escobar, Qiao, Craft and Phelps2021; Rothlisberger and Lodge Reference Rothlisberger and Lodge2011; Zipp et al. Reference Zipp, Lewis, Provencher and Vander Zanden2019).
A study conducted in Minnesota concluded that M. spicatum reaches high abundances under conditions also favored by other common North American aquatic plants, such as pondweeds (Potamogeton species), which may indicate competitive superiority and greater likelihood of driving native plant decline via competitive exclusion (Verhoeven et al. Reference Verhoeven, Glisson and Larkin2020). The plant also interferes with boating, swimming, and sport fishing (Nichols and Lathrop Reference Nichols and Lathrop1994; Smith and Barko Reference Smith and Barko1990; Tamayo and Olden Reference Tamayo and Olden2014; Verhofstad and Bakker Reference Verhofstad and Bakker2019). A few studies have shown that riparian property values are negatively correlated with the presence of M. spicatum and may be reduced by 1% to 16% (Horsch and Lewis Reference Horsch and Lewis2009; Liao et al. Reference Liao, Wilhelm and Solomon2016; Zhang and Boyle Reference Zhang and Boyle2010). This reduction could impact revenues of small municipalities, as the development of vacant lots may be less likely near invaded lakes (Goodenberger and Klaiber Reference Goodenberger and Klaiber2016).
Citizens living near infested lakes are directly impacted by M. spicatum invasions. They are typically the instigators of control measures, with or without governmental or scientific support. The objective of control is not eradication, but rather impact mitigation through substantial biomass reduction. In the United States, laws and regulations permit the use of herbicides in lakes, and a wide range of products are available. In Canada, the regulatory context is much less permissive regarding the spraying of herbicides in water, and few products are available. Environmental managers must therefore rely on mechanical methods such as mowing or physical methods like hand pulling or benthic matting.
Hand pulling is a straightforward control method. The plant is manually extracted by divers, taking care to remove not only the stems, but also the shallow root system. The harvested material is then brought to the surface in bags or using a suction device (Bailey and Calhoun Reference Bailey and Calhoun2008; Boylen et al. Reference Boylen, Eichler and Sutherland1996; Eichler et al. Reference Eichler, Bombard, Sutherland and Boylen1993; Kelting and Laxson Reference Kelting and Laxson2010; Shaw et al. Reference Shaw, Hymanson and Sasaki2016). Large-scale hand-pulling campaigns have been undertaken at Lake George and Upper Saranac Lake in New York State. At Lake George (area: 110 km2), from 1986 to 2020, more than 40,000 person-hours of diving with suction harvesting permitted the removal of 675,000 kg of M. spicatum. By late summer 2021, M. spicatum beds were only found at 33 of 217 sites where they had initially been recorded. Although the control efforts were effective, they required a colossal investment of more than US$5.5 million. Unfortunately, certain sites were reinfested due to inadequate monitoring, and in 2020, 95,000 kg of M. spicatum were removed, followed by 68,000 kg in 2021 to prevent the situation from deteriorating further. Discouraged, local managers began questioning their approach and concluded that herbicides should be used instead to reduce costs (AE Commercial Diving Service 2021; Gagné Reference Gagné2021). The control campaign at Upper Saranac Lake (19 km2), initiated in 2004, resulted in the removal of more than 22,000 kg of M. spicatum in the first 3 yr, an effort of nearly 35,000 person-hours of diving. This effort reduced M. spicatum biomass by 97%. The initial intensive control phase was followed by a maintenance phase that, in recent years, has yielded a very small summer harvest (20 kg in 2022). Although this project is exemplary in terms of sustainability, it has nevertheless required a total expenditure of more than US$2 million (Middleton Reference Middleton2022).
In Emerald Bay (1.9 km2) of Lake Tahoe (502 km2) in California, Shaw et al. (Reference Shaw, Hymanson and Sasaki2016) demonstrated that with proper planning, M. spicatum can be eliminated, at least in the short term, by combining benthic matting and hand pulling. Benthic matting is a nonselective control method (Helsel et al. Reference Helsel, Gerber and Engel1996; Laitala et al. Reference Laitala, Prather, Thill, Kennedy and Caudill2012). Also called benthic barriers or screens, benthic mats are made of fiberglass or jute. They do not kill the plants by depriving them of light, but rather act as physical barriers to their growth (Mayer Reference Mayer1978; Perkins et al. Reference Perkins, Boston and Curren1980). In Emerald Bay, the initial intensive phase (matting + hand pulling) lasted 4 yr and eliminated 2.4 ha of M. spicatum. The maintenance phase (hand pulling only), of indefinite duration, is expected to prevent reinvasion. In 2018, for example, monitoring resulted in the rapid detection and removal of 60 M. spicatum individuals, even though no specimen had been detected in the previous 4 yr (Hauge Brueck Associates, LLC Reference Hauge Brueck Associates2020). The main conclusions of Shaw et al. (Reference Shaw, Hymanson and Sasaki2016) are (1) a large initial investment is required, (2) costs decrease over time if the initial stage is successful and followed by annual monitoring, (3) physical techniques can reduce the biomass of an invasive plant relatively quickly if adequate effort is made, and (4) focusing control efforts on a small number of sites is more effective than thinly stretching resources over a broad area.
These examples show that M. spicatum can be controlled without herbicides. However, large-scale experiments, such as those of Lake George and Upper Saranac Lake, are unlikely to become widespread without state or provincial financial support because of their prohibitive costs. The outlook is more encouraging for small lakes with only a few hectares of M. spicatum beds. Nevertheless, although the Emerald Bay project was successful, more case studies are necessary to convince managers that effective alternatives to herbicides exist. In this study, we hypothesized that M. spicatum control could be accomplished rapidly and at a reasonable cost by systematizing the use of fiberglass benthic mats supplemented by hand pulling. Our objective was to reduce the coverage of M. spicatum by 95% within 5 yr in a small lake of about 1 km2 (Lac des Abénaquis), which represents the size of most of the invaded lakes in Québec (Canada). In addition to rigorously evaluating the effectiveness of benthic matting and hand pulling, we also sought to precisely quantify the time invested in order to determine the conditions under which benthic matting is more effective than hand pulling, from an economic standpoint. Finally, we used M. spicatum fragmentation as an additional indicator of control success, by collecting fragments that had washed up on the lakeshore.
Materials and Methods
Study Area
This study was conducted at Lac des Abénaquis (46.165833°N, 70.361944°W), located in the municipality of Sainte-Aurélie (population: 847), in southern Québec, about 5 km from the Québec–Maine border. The area of the lake is 1.2 km2, and its maximum water depth is 4 m. Its watershed has a total area of 43 km2. The closest weather station is located in the town of Saint-Prosper (46.212778°N, 70.479167°W), 10 km from Sainte-Aurélie. At this station, the mean annual temperature is 4 C, January being the coldest month (mean temperature: −13 C) and July the warmest month (18 C). Total annual precipitation averages 1,117 mm, of which 22% falls as snow (Ministère de l’Environnement et de la Lutte contre les changements climatiques, de la Faune et des Parcs du Québec 2020).
Lac des Abénaquis is surrounded by nearly 200 permanent and seasonal residences connected to the municipal sewer system since 1980. The municipality acquired a watercraft cleaning and inspection station in 2012, which boaters and fishers must use before accessing the lake’s boat launch. Despite this measure, the lake has been invaded by M. spicatum since at least 2013. By 2016, M. spicatum covered more than 3.6 ha (Paradis and Jacques Reference Paradis and Jacques2016). That same year, the local association of shoreline residents undertook a control project based on hand pulling and benthic matting with fiberglass screens.
Repeated mapping of M. spicatum beds (Figure 1) revealed that they shrank from 3.6 ha in 2016 to about 1 ha in 2019. Citizen efforts (Table 1) therefore appear to have been effective. However, because untreated sites were not monitored, it is unknown whether this reduction was caused by control efforts or simply a consequence of natural decline, which often occurs in M. spicatum populations (Kujawa et al. Reference Kujawa, Frater, Mikulyuk, Barton, Nault, Van Egeren and Hauxwell2017). It was not until 2020 that a rigorous monitoring plan was established.

Figure 1. Evolution of Myriophyllum spicatum patches in Lac des Abénaquis (Québec, Canada) from 2014 to 2021.
Table 1. Myriophyllum spicatum control efforts conducted at Lac des Abénaquis (Québec, Canada) from 2016 to 2021.

Control Strategy
Theoretically, a higher initial control effort (1) results in a lower chance of reinvasion and (2) helps in attaining the less demanding maintenance phase sooner (Baker and Bode Reference Baker and Bode2016; Hussner et al. Reference Hussner, Nehring and Hilt2014; Kaiser and Burnett Reference Kaiser and Burnett2010; Kelting and Laxson Reference Kelting and Laxson2010; Larson et al. Reference Larson, Phillips-Mao, Quiram, Sharpe, Stark, Sugita and Weiler2011; Odom et al. Reference Odom, Cacho, Sinden and Griffith2003; Shaw et al. Reference Shaw, Hymanson and Sasaki2016). At Lac des Abénaquis, the control strategy planned for 2020 and 2021 sought to maximize the available resources (workforce and equipment), while minimizing the risk of propagating M. spicatum. The control strategy was devised to sharply reduce the biomass of the invader, thus curbing its impacts on biodiversity and lake users. In 2020, the association of shoreline residents deemed that the presence of M. spicatum in the lake was acceptable insofar as the total infested area did not exceed 1,000 m2.
To achieve this goal, in 2020, we prioritized the largest and densest M. spicatum patches in the lake. Large, dense patches generate many diaspores (Blackwood et al. Reference Blackwood, Hastings and Costello2010; Taylor and Hastings Reference Taylor and Hastings2004), and control efforts should therefore target them first (Baker Reference Baker2017; Hastings et al. Reference Hastings, Hall and Taylor2006; Hulme Reference Hulme2003; Jarnevich and Stohlgren Reference Jarnevich and Stohlgren2009). Selection of subsequent patches for priority treatment was based on their proximity to the largest patches, given that concentrating resources on fewer sites is more effective than dispersing efforts over multiple scattered sites (Shaw et al. Reference Shaw, Hymanson and Sasaki2016). In parallel, isolated M. spicatum plants were also targeted in 2020 for removal before they developed into new patches. We reasoned that eliminating isolated individuals would be cost-effective over the long term by preventing the formation of new beds that not only generate many fragments, but are also energy-intensive to control.
This strategy was implemented using two distinct treatments, benthic matting and hand pulling. Benthic matting, with supplementary hand pulling around the mat edges, was chosen for patches >100 m2. Aquascreen® brand fiberglass mats (Traitements Bio-Bac, Sherbrooke, Québec, Canada) were chosen for this project. Each mat measured 2 by 15 m and had a mesh size of 65 perforations cm−2, which permits gas exchange and thus prevents billowing (Engel Reference Engel1983; Mayer Reference Mayer1978; Perkins et al. Reference Perkins, Boston and Curren1980). About 5,200 and 6,240 m2 of fiberglass mats were available to cover M. spicatum beds in 2020 and 2021, respectively. An 8- to 10-wk matting period usually results in 100% destruction of individuals if the mats are properly installed (Laitala et al. Reference Laitala, Prather, Thill, Kennedy and Caudill2012; Perkins et al. Reference Perkins, Boston and Curren1980). Fiberglass mats are reusable; the manufacturer claims a lifespan of 10 to 15 yr, although mats can tear and require repair.
The benthic mats were set out from June 1 to 6 (2020) and from May 24 to June 8 (2021). The mats were removed approximately 10 wk after installation (2020: August 10 to 28; 2021: August 8 to 19). They were then cleaned, repaired if necessary, and stored. The benthic matting operations (installation, removal, cleaning, repairing) were timed to track the work hours. Installation and removal included travel time on the water and pre-dive preparation.
Isolated plants and patches <100 m2 were eliminated with hand pulling by divers. The harvested material was brought to the surface via a suction hose or in hand-filled bags. For dense patches, it was quicker and more efficient to use a suction system to bring the plant material to the surface through a hose handled by a diver. The suction system comprised a floating platform equipped with an outboard motor, a mechanical pump to generate suction, and a sieve at the hose outlet to retain the harvested material while allowing water to drain through. Although the divers worked in teams of two, which is essential for safety reasons, only one could use the suction hose at a time. The second diver thus filled a bag with plants and surfaced it. An assistant on the platform received the bags as they were brought topside. Removal of M. spicatum by hand was done from June 17 to August 17 (2020) and from June 2 to August 9 (2021). For each harvesting session, the location, dive time, and fresh weight of M. spicatum were recorded, while distinguishing between plants brought to the surface using the suction system versus those brought up in bags.
Monitoring the Efficacy of Myriophyllum spicatum Control
A robust monitoring protocol was implemented to evaluate the success of the control program. First, a sampling plan based on quadrats was established to evaluate the stem density of M. spicatum in 2020 and 2021. Second, the M. spicatum patches were mapped at the end of each summer to detect any changes in surface area. Finally, M. spicatum stem fragments were collected along the shoreline to assess the extent of plant fragmentation from mid-July to late August.
Sampling points were systematically generated—one point every 50 m2—in Lac des Abénaquis using the Add a Grid tool of the ArcGIS geographic information system (v. 10.8.1., Environmental Systems Research Institute, Redlands, CA, USA). Three different zones were then delineated in the lake using the August 2019 M. spicatum mapping as a baseline (VG, unpublished data), namely, (1) areas where M. spicatum was present with high density (≥25% of lake bottom covered); (2) areas where M. spicatum was present with low density (<25%); and (3) areas where M. spicatum was absent, but at the depths where the plant would typically be found in the lake, that is, between 1 and 2.5 m. In each of these three zones, 60 sampling points were randomly selected from all the generated grid points using the SelectRandomByCount command in the Python window in ArcGIS.
The sampling points were first visited in 2020 from June 1 to 17 (435 accumulated degree days >0 C by June 1), before the benthic mat installation. Using a geographic positioning system (GPS), the team boated to each of the selected points. On-site, a rebar quadrat measuring 0.25 m2 was sunk. The divers then descended to count the number of M. spicatum stems in the quadrat in addition to stems of other plants identified to the genus or species level. The same process was repeated in 2021 from May 21 to June 1 (435 accumulated degree days >0 C by May 21), at the same locations, although the quadrat may have settled in slightly offset locations between 2020 and 2021. Stem density data collected in 2021 were compared with those of 2020 with paired-sample Wilcoxon signed-rank tests, because the data were not normally distributed (Scherrer Reference Scherrer1984).
The patches of M. spicatum were mapped in late summer 2020 (August 12 to 28) and 2021 (August 6 to 20). The mapping was conducted by a team of three using an aquascope, a GPS, and a floating platform. The littoral was surveyed by making return trips from the shoreline to the center of the lake, with about 10 m between each round trip. Where several plants grew together (>10 individuals) and formed a patch, the patch perimeter was circumscribed by circling the area with the platform while recording GPS points. Where plants grew alone, a single point was taken. The delineations and points were then imported into ArcGIS software to map both the patches and the isolated stems.
Washed-up stem fragments of M. spicatum were collected by three people as they walked the perimeter of the lake, between mid-July and late August, which is when the plant produces most of its fragments in the Northern Hemisphere (Madsen et al. Reference Madsen, Eichler and Boylen1988; Madsen and Smith Reference Madsen and Smith1997; Smith and Barko Reference Smith and Barko1990). In 2020, fragments were collected on July 21 to 22 (1,350 degree days >0 C), August 3 to 4 (1,620), and August 24 to 26 (2,000). In 2021, collections were made on July 20 (1,440), August 2 (1,640), and August 23 to 24 (2,100). The number of fragments was counted, and the total length of each fragment measured. The presence of roots was also noted.
Results and Discussion
Benthic matting
The use of 1,000 m2 of benthic mats required an average of 51 (2020) and 47 (2021) person-hours. This includes installation (15 to 18 person-hours), removal (10 to 11), and maintenance (21 to 23). Maintenance was the most effort-intensive step, as each mat required washing before storage. Also, 20% of the mats were torn and had to be repaired. Maintenance is often overlooked in work time budgets relating to the use of fiberglass mats, yet this aspect alone accounted for 40% to 50% of the labor budget.
Hand Pulling
Hand pulling required a total of 103 (2020) and 140 (2021) person-hours, and allowed the removal of 1,015 (2020) and 1,230 (2021) kg of fresh M. spicatum biomass. Removal with bags (190 person-hours) required more time than removal with the suction system, but the biomass brought to the surface was 2.4 times higher per person-hour with the suction system than with the bags. The reason divers did not use the suction system more often is because when the density of M. spicatum stems is low, the search time is high. Divers must cover great distances to find and remove the scattered plants. Therefore, they often choose to use bags rather than the suction system to reduce energy expenditure and oxygen use, as it is physically demanding to tow the floating platform by the suction hose. The decision threshold for using the suction system, set subjectively by the divers, but nevertheless based on 2 yr of experience, was 10 kg of fresh biomass removed per person-hour of diving. Above this threshold, the suction system was assumed to be more efficient than bagging.
Monitoring the Efficacy of Myriophyllum spicatum Control
The average density in spring 2020 of patches with a high M. spicatum cover was 58 stems m−2. Native species [Canadian waterweed: Elodea canadensis Michx., nodding waternymph: Najas flexilis (Willd.) Rostk. & Schmidt, Robbins’ pondweed: Potamogeton robbinsii Oakes] were noted in only 8 of the 60 quadrats. For patches with a low cover, the average density was 10 stems m−2. Native species (E. canadensis, quillworts: Isoetes sp., alternateflower watermilfoil: Myriophyllum alterniflorum DC., N. flexilis, P. robbinsii) were noted in 25 of the 60 quadrats. In areas where M. spicatum had been presumed absent, the invader was detected in only 2 of the 60 quadrats, whereas native species (E. canadensis, Isoetes sp., M. alterniflorum, N. flexilis, P. robbinsii) were observed in 30 of the 60 quadrats. Overall, the stem density of M. spicatum (Table 2) dropped sharply and significantly between the spring of 2020 and the spring of 2021 where control measures were applied (benthic matting, hand pulling). In contrast, M. spicatum density strongly and significantly increased in the absence of intervention, while that of native plants remained stable.
Table 2. Stem density of Myriophyllum spicatum and native plants in Lac des Abénaquis (Québec, Canada) under different management regimes.
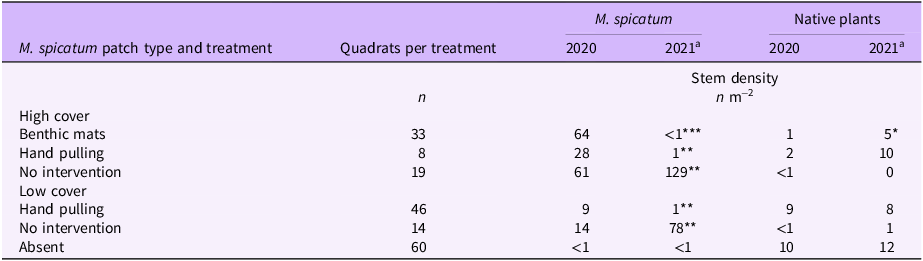
a 2021 significantly different from 2020: *P < 0.05; **P < 0.01; ***P < 0.001.
Following the treatment of dense M. spicatum beds with benthic mats, stem density was reduced to almost nothing (99% reduction). After the mats were removed in August 2020, native vascular plant density increased, and by the next spring (May to June 2021), they were occupying the space formerly filled by M. spicatum. Before the benthic mats, native vascular plants were observed in only 6% of the monitored quadrats. Post-matting, this percentage increased to 70%. The matted sites were thus significantly revegetated by native plants (Table 2), a phenomenon also noted by other researchers (Boylen et al. Reference Boylen, Eichler and Sutherland1996; Eichler et al. Reference Eichler, Bombard, Sutherland and Boylen1995). The proximity of native plant patches and the presence of a seed reservoir in the substrate likely accelerated this recovery. A return of native vegetation should help overcome the reluctance of managers who believe that benthic matting is environmentally damaging due to a low selectivity. In reality, the nonselective vegetation suppression is only temporary.
The average stem density in M. spicatum beds that were only hand pulled was reduced by 96% following the first treatment. The hand-pulling results obtained at Lac des Abénaquis are consistent with those of Lake George and Upper Saranac Lake, where an 86% to 94% drop in M. spicatum stem density was observed following control operations (Eichler et al. Reference Eichler, Bombard, Sutherland and Boylen1993; Kelting and Laxson Reference Kelting and Laxson2010). While hand pulling did not significantly increase the stem density of native plants, it probably helped to maintain their populations (Bailey and Calhoun Reference Bailey and Calhoun2008; Eichler et al. Reference Eichler, Bombard, Sutherland and Boylen1993; Nicholson Reference Nicholson1981; Shaw et al. Reference Shaw, Hymanson and Sasaki2016).
The surface area of M. spicatum patches (Figure 1) treated with benthic matting and/or hand pulling decreased from 6,300 m2 (June 2020) to 335 m2 (August 2020), a 95% reduction. Conversely, untreated M. spicatum patches increased by 111%, expanding from 3,300 to 6,965 m2 during the same period. No M. spicatum patches were observed in August 2021, as these were totally eliminated by the matting and hand pulling during summer 2021. Only 560 widely scattered plants remained. The control objective established in the spring of 2020 was therefore fully achieved.
To our knowledge, this is the first study to precisely document M. spicatum fragmentation in a lacustrine environment. In both 2020 and 2021, the number of M. spicatum fragments washed up on the lakeshore increased markedly as the summer progressed (Table 3). Although the number of beached fragments showed a dramatic 63% decrease from 2020 to 2021, the number found was substantial despite the effectiveness of control measures. Between 26% and 28% of the fragments had roots, which would increase their chances of generating new beds should they settle to the lake bottom. These washed-up fragments indicate that control efforts are far from over: even though all the M. spicatum beds were eliminated, remaining scattered plants continue generating fragments that could spawn new infestations. It is therefore essential to implement a long-term maintenance strategy, which will mainly involve annual hand pulling.
Table 3. Myriophyllum spicatum stem fragments found in 2020 and 2021 on the shores of Lac des Abénaquis (Quebec, Canada) by harvest date.

The Lake George experience clearly shows the importance of maintaining an annual monitoring and hand-pulling campaign at sites where M. spicatum could reestablish, in order to protect the investments made in the initial intensive management phase. For example, a site that could easily have been treated in 1986, when about 10 plants were counted (Eichler and Boylen Reference Eichler and Boylen1986), had formed a dense 800 m2 bed within 2 yr. A rapid response (hand pulling) on such sites is much less costly than intervening a few years later with benthic mats (Madsen et al. Reference Madsen, Sutherland, Eichler and Bombard1991).
Ultimately, a herbicide-free control campaign for M. spicatum requires a considerable investment in the first few years to massively reduce the biomass of the invader. Unfortunately, the funds available for control are rarely sufficient to tackle an entire population over a short period. If the available budget is insufficient or long-term unsustainable, a control campaign is likely to fail (Boylen et al. Reference Boylen, Eichler and Sutherland1996; Eichler et al. Reference Eichler, Bombard, Sutherland and Boylen1995; Kelting and Laxson Reference Kelting and Laxson2010; Shaw et al. Reference Shaw, Hymanson and Sasaki2016). For example, at Upper Saranac Lake, before intensive hand-pulling was initiated in 2004, a budget of US$55,000 was allocated annually from 1999 to 2003 for the control of M. spicatum; this budget was insufficient, and the beds continued to expand. Beginning in 2004, an annual budget of approximately US$350,000 yielded a substantial and, more importantly, sustainable reduction of the invader biomass (Kelting and Laxson Reference Kelting and Laxson2010).
The example of Lac des Abénaquis, whose small lakeside population is less than 1,000, demonstrates that an invasion of M. spicatum can successfully be managed at an acceptable cost. The cost acceptability of a control project may, of course, vary greatly between communities. The citizens of Sainte-Aurélie were strongly motivated to reverse the M. spicatum invasion. The leaders of the local association of shoreline residents were creative in securing funding from various sources and mobilizing scientific expertise. They were highly supported by the municipality. At Lac des Abénaquis, intensive management (years 1 to 5) using benthic mats and hand pulling cost an estimated Can$185,000 (US$145,000) ha−1 of M. spicatum beds, including an initial investment of Can$60,000 (US$45,000) for the purchase of the fiberglass benthic mats. Maintenance management (years 6+) with hand pulling was estimated at about Can$20,000 (US$15,000) per year. These are upper estimates, as they involve the use of a for-profit company, but the estimation for the maintenance phase is very close to reality (summer seasons 2022 and 2023; C Maranda, Association des riverains du lac des Abénaquis, personal communication). Volunteer labor and in-kind contributions (employees, materials) provided by a municipality can significantly lower these costs, up to −40% for the specific case of Lac des Abénaquis (Gagné Reference Gagné2021).
While encouraging, effective control of M. spicatum nevertheless remains expensive, even with the contributions of volunteers and the support of various levels of government. The high costs deserve serious consideration, as the same money could be used elsewhere to address more pressing environmental issues undermining lacustrine health. At Lake George, the cost of physical control (hand pulling) was recently contrasted with that of a herbicide (florpyrauxifen-benzyl, ProcellaCOR™; SePRO Corporation, Carmel, IN, USA) presumed to be specific and safe and having a long-lasting effect. Although there is little field-based evidence for these assumptions (Beets et al. Reference Beets, Heilman and Netherland2019; Bloodsworth Cattoor et al. Reference Bloodsworth Cattoor, Londo, Walsh and Lund2022; Haug et al. Reference Haug, Ahmed, Gannon and Richardson2021; Princeton Hydro, LLC Reference Princeton Hydro2021) the projected cost of herbicide application is highly competitive. In 2022, hand pulling at Lake George cost US$360,000, while a herbicide pilot project was estimated at about US$39,000 for approximatively the same area treated (Craig Reference Craig2023). Heated debates arose between the Adirondack Park Association and the Lake George Park Commission (LGPC), the promoters of the herbicide option, and the local association of shoreline residents, who felt that there were too many unknowns concerning the impacts of the pesticide on non-target species and human health. In 2023, the New York State Supreme Court upheld the initial injunction, which had halted the herbicide use in 2022, essentially because of an incomplete consultation and approval process. Judge Robert Muller nevertheless stated: “Although the DASH [diver assisted suction harvester] management program is certainly an alternative for management of EWM [Eurasian watermilfoil] in Lake George, there is no dispute that ProcellaCOR™ is far more cost effective—especially when considering a body of water as large as Lake George that must triage its resources. In this regard, the conclusion that ProcellaCOR is the only alternative reasonably able to accomplish the LGPC’s objective—namely the eradication of EWM at a lower cost—is not irrational” (New York State Supreme Court 2023). This example clearly illustrates the importance of cost when evaluating options for aquatic plant control.
Acknowledgments
This research was financially supported by the Natural Sciences and Engineering Research Council of Canada (grant to CL), the Association des riverains du lac des Abénaquis (Claire Maranda), the Municipalité of Sainte-Aurélie, the Fondation de la Faune du Québec, and the Caisse Desjardins du Sud de la Beauce. The authors thank Charlotte Bergeron, Jacob Bouchard, Étienne Fortin, Maxim Gagné, Danick Landry, and especially Théodore Paquet, for field assistance; Elisabeth Groeneveld for the English translation of the manuscript; and Daniel Larkin, Ryan Wersal, and an anonymous reviewer for comments. No conflicts of interest have been declared.







