Published online by Cambridge University Press: 20 January 2017
Soil engineering by downy brome may be a facet of its competitiveness. Using rhizotrons in the greenhouse, we compared the growth and plant–soil relationships of downy brome grown in two field soil types: soil invaded for 12 yr by downy brome and a similar soil not yet invaded. For each soil type, downy brome was grown for two growth cycles. At harvest, root mass and soils were sampled at depths of 10, 40, and 80 cm (4, 16, and 32 in); aboveground biomass was also sampled. After the first growth cycle, downy brome grown in invaded soil had 250% greater aboveground biomass and nearly double the root mass per soil volume at 10 cm relative to downy brome grown in noninvaded soil; root mass per volume was similar at depths of 40 and 80 cm. For the second growth cycle, aboveground biomass declined, but was twice greater for downy brome grown in invaded soil; however, root mass per volume was similar between soil types for each depth. Soil attributes that positively related to aboveground biomass included bicarbonate-extractable P, DTPA (diethylentriamene pentaacetate)-extractable Mn, and solution-phase 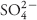 (80-cm depth). We conclude that the data support our hypothesis that downy brome has engineered the soil to increase its growth potential, but proof will require a more robust experimental design. Plant competition is affected by myriad interactions; however, a plant that can increase the availability of soil nutrients for itself and its growth potential, relative to competing plants, would appear to be at an advantage. The mechanistic underpinnings involved are inconclusive, but may involve increased availability of soil N, P, and Mn.
(80-cm depth). We conclude that the data support our hypothesis that downy brome has engineered the soil to increase its growth potential, but proof will require a more robust experimental design. Plant competition is affected by myriad interactions; however, a plant that can increase the availability of soil nutrients for itself and its growth potential, relative to competing plants, would appear to be at an advantage. The mechanistic underpinnings involved are inconclusive, but may involve increased availability of soil N, P, and Mn.