Introduction
Behavioral and psychological symptoms of dementia (BPSD; also referred to as neuropsychiatric symptoms, NPS) are common manifestations of Alzheimer's disease (AD) as well as vascular dementia (VaD; Lyketsos et al., Reference Lyketsos, Steinberg, Tschanz, Norton, Steffens and Breitner2000). They adversely affect the patients' quality of life (Karttunen et al., Reference Karttunen2011), cause distress to caregivers (Cummings and McPherson, Reference Cummings and McPherson2001), increase the risk for nursing home placement (Tun et al., Reference Tun, Murman, Long, Colenda and von Eye2007), and increase the cost of care (Jönsson et al., Reference Jönsson2006). The presence of BPSD has also been reported to predict the progression to severe dementia in AD. Whereas in older diagnostic systems (McKhann et al., Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan1984; Román et al., Reference Román1993) such symptoms were classified as merely “consistent” with a diagnosis of AD or VaD, they are now listed among the “core clinical criteria” for all-cause dementia (McKhann et al., Reference McKhann2011), reflecting a changing view about the clinical significance of these symptoms. BPSD are therefore a major target for treatment in patients with dementia. Beneficial effects have been reported for some anti-dementia drugs (cholinesterase inhibitors, memantine), anti-depressants (Gauthier et al., Reference Gauthier2010), and some antipsychotics (Tan et al., Reference Tan2015). Specific effects of citalopram in patients with agitation associated with AD have been reported (Porsteinsson et al., Reference Porsteinsson2014).
A recently published systematic review (von Gunten et al., Reference von Gunten, Schlaefke and Überla2016) identified four large, randomized, placebo-controlled, double-blind, multi-center trials of the defined, quantified Ginkgo biloba extract EGb 761® in patients with mild to moderate dementia who had clinically significant BPSD. (EGb 761® is a dry extract from Ginkgo biloba leaves (35–67:1), extraction solvent: acetone 60% (w/w). The extract is adjusted to 22.0%–27.0% ginkgo flavonoids calculated as ginkgo flavone glycosides and 5.0%–7.0% terpene lactones consisting of 2.8%–3.4% ginkgolides A, B, C, and 2.6%–3.2% bilobalide and contains less than 5 ppm ginkgolic acids.) In two 22-week trials, the Neuropsychiatric Inventory (NPI; Cummings, Reference Cummings1997) was used as secondary outcome measure, showing significant superiority of EGb 761® over placebo in one trial and a tendency favoring active treatment in the other (Napryeyenko et al., Reference Napryeyenko and Borzenko2007; Nikolova et al., Reference Nikolova, Yancheva, Raychev and Hoerr2013). In two 24-week trials, change in the NPI composite score was prospectively defined as co-primary outcome and the caregiver distress score was a secondary outcome. In both trials, EGb 761® was significantly superior to placebo in composite scores as well as caregiver distress ratings (Ihl et al., Reference Ihl2011; Herrschaft et al., Reference Herrschaft, Nacu, Likhachev, Sholomov, Hoerr and Schlaefke2012).
The objective of this meta-analysis was to examine the treatment effects of EGb 761® on single behavioral and psychological symptoms with regard to overall reduction of symptoms, reduction of symptoms present at baseline, the prevention of newly emerging symptoms and implications for caregiver burden.
Patients and methods
Eligibility criteria, data sources, and search
Randomized, placebo-controlled clinical trials of EGb 761® with a minimal duration of 20 weeks, including patients with dementia (probable AD, probable VaD or possible AD with cerebrovascular disease) who had clinically significant BPSD (NPI total score at least 6) were identified.
We used a sensitive search strategy including the following electronic databases: PubMed, including MedLine (from beginning to December 2013), EMBASE (from January 2006 to December 2013), and PASCAL (from beginning to December 2013). The following search terms were used (with * characterizing a wildcard, and the items AND and OR being used as Boolean functions): (ginkg* OR gingk*) AND clinical trial(pt) for PubMed including MedLine, ((ginkg* OR gingk*) NOT medline(sb)) AND (clinical* OR trial OR randomized) for PubMed excluding Medline, (GINKGO OR GINGKO), AND (HUMAN/CT OR HOMME/CTFR) for PASCAL, and (ginkgo or gingko) AND CT = (CLINICAL TRIAL; CLINICAL STUDY; DOUBLE BLIND PROCEDURE) AND py > 2005 for EMBASE. A search update was conducted in June 2016. Furthermore, the reference sections of systematic reviews were screened for primary publications. In addition, the manufacturer of EGb 761®, Dr. Willmar Schwabe GmbH & Co. KG, was asked about studies that were not identified by the search or had not been published when the search was performed.
Selection and critical appraisal of clinical trials
Two persons independently from each other screened the results of the literature search and the retrieved full papers for eligibility. Their judgments were concordant; no discrepancies had to be resolved. Trial and publication quality were assessed in terms of randomization and allocation concealment, blinding of patients and investigators, sample size estimation, proportion of trial discontinuations, application of the intent-to-treat principle and whether statistical analyses were reported and judged adequate. All included trials adequately met these quality criteria.
Four eligible studies were identified by the original database search; no further relevant study was identified by the search update or by the manufacturer. The four trials were randomized, placebo-controlled, double-blind, multi-center, parallel-group trials performed independently of each other in adherence to the provisions for Good Clinical Practice (GCP) set by the International Conference on Harmonization and the World Medical Association's Declaration of Helsinki. For all trials, approval by regulatory bodies and ethics committees was obtained and patients were enrolled only after written informed consent had been received. The methods and main results have been reported in the primary publications in peer-reviewed journals (GINDEM-NP Study: Napryeyenko et al., Reference Napryeyenko and Borzenko2007; GOTADAY Study: Ihl et al., Reference Ihl2011; GOT-IT! Study: Herrschaft et al., Reference Herrschaft, Nacu, Likhachev, Sholomov, Hoerr and Schlaefke2012; PLAGIN Study: Nikolova et al., Reference Nikolova, Yancheva, Raychev and Hoerr2013).
All four trials were similarly designed and enrolled patients with mild to moderate dementia who scored 35 or less in the Test for the Early Detection of Dementia with Differentiation from Depression (Mahoney et al., Reference Mahoney, Johnston, Katona, Maxmin and Livingston2005) and 9 to 23 on the SKT short cognitive performance test (Kim et al., Reference Kim, Nibbelink and Overall1993) and had clinically significant BPSD, as shown by a composite score of at least 6 on the NPI, with at least one item score (other than delusions or hallucinations) of at least 3. Diagnoses of probable AD, probable VaD or possible AD with cerebrovascular disease were established using the research diagnostic criteria by McKhann et al. (Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan1984) and/or Román et al. (Reference Román1993), as applicable. In accordance with random allocation, patients received Ginkgo biloba extract EGb 761® at a daily dose of 240 mg or matching placebo tablets for periods of 22 weeks (GINDEM-NP, PLAGIN) or 24 weeks (GOTADAY, GOT-IT!). The primary outcome measure in all studies was the change in the SKT total score. The change in the NPI composite score was co-primary in the two 24-week trials and a secondary outcome in the 22-week trials. Scales for activities of daily living, global ratings, and quality-of-life scales (in 24-week trials only) were recorded as secondary outcomes.
Outcomes
Outcomes of interest for this meta-analysis were the single items of the NPI. The NPI is a 12-item inventory to assess the presence and severity of behavioral changes in patients with dementia. The ratings of frequency, severity, and related caregiver distress are based on an interview of the clinician with the patient's caregiver. The twelve symptoms/disorders assessed are delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, sleep and nighttime behavior disorders, appetite and eating disorders. Each symptom/disorder is rated by frequency (range: 1 to 4) and severity (range: 1 to 3); a composite score is formed by multiplying the scores for frequency and severity (range: 1 to 12). In addition, caregiver distress is rated along a scale ranging from 0 to 5. Higher scores indicate more impairment.
Statistical analysis
For all selected trials, individual participant data were provided by Dr. Willmar Schwabe GmbH & Co KG. For each of the four clinical trials the effects of EGb 761® were compared to placebo with respect to the total composite score, the total caregiver distress score and their respective 12 single items. For the overall reduction of symptoms and the reduction of symptoms present at baseline (including patients with symptoms in the respective item at baseline), an analysis of covariance (ANCOVA) model was used to calculate the changes from baseline to week 22/24. The ANCOVA model included terms for treatment group and baseline value of respective outcome variable as covariates (stage 1). The resulting least square means (LSM) and standard deviations of each study were combined in a fixed effect meta-analysis (summary measure: weighted difference in LSM (LSMD) and 95% confidence limit for the changes from baseline to 22/24 week follow-up for the EGb 761® treatment group compared to the placebo group; negative LSMDs show an advantage of the EGb 761® treatment group; stage 2). The fixed effects model using the inverse variance method was chosen due to the similar design, nearly identical inclusion and exclusion criteria, daily doses, and duration of treatment of the four included studies.
Furthermore, for each study, the incidence of newly emerging symptoms (represented by the respective item of the NPI, separately for each item) from baseline to 22/24 week follow-up was calculated under EGb 761® and placebo in patients with a baseline value of 0 for the respective symptom (i.e. respective symptom not present at baseline; stage 1). To assess the potential for prevention of newly emerging symptoms by EGb 761® compared to placebo, weighted relative risks (RR) and 95% CI were calculated by a meta-analysis for binary data using a fixed effects model to combine results of the four clinical trials (EGb 761® treatment compared to placebo; stage 2). RR below 1 shows an advantage of the EGb 761® treatment group.
To assess the clinical relevance of treatment effects of EGb 761® for the twelve single items of the NPI (each for composite and caregiver distress scores), standardized mean differences (SMD) were calculated for the results of the meta-analyses (SMD = LSMD/standard deviation) in those patients in whom the respective symptoms were present at baseline. Cohen (1992) defined an SMD of 0.5 (reflected by the interval from 0.4 to 0.6) as medium effect size, SMDs below 0.4 as small and SMDs above 0.6 as large effects.
All outcomes were assessed in the intention-to-treat/full-analysis sets. The last observation during randomized treatment was carried forward (LOCF) for missing values of the outcome variables on a single item basis. The maximum of missing values replaced by LOCF for a single item was 3.2%.
The meta-analyses were performed using the package “meta” in R (Version 3.1.0). As all items have equal weight in the composite and caregiver score, no item was selected as primary outcome. In all analyses, statistical significance was assumed if p < 0.05 as adjustment for multiple testing is not routinely used in meta-analyses.
Results
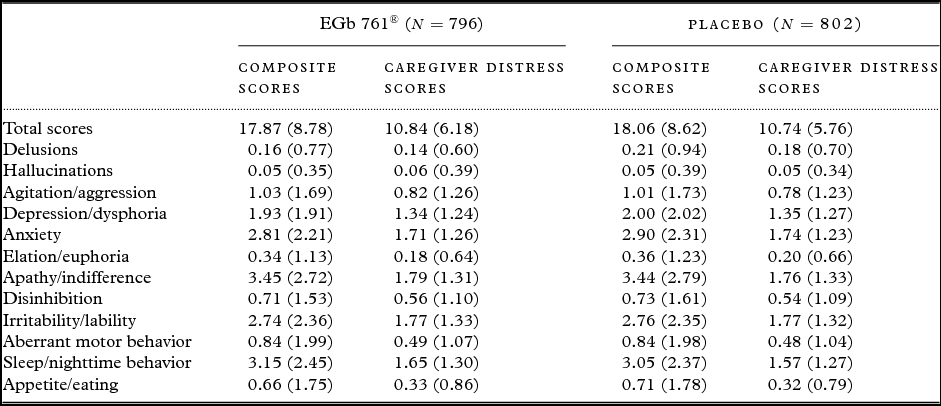
A total of 1628 patients were randomized to the four trials. Of these, 1,598 patients (98%) had efficacy data after baseline (EGb 761®, 796; placebo 802). At baseline, there were no conspicuous differences between treatment groups with respect to demographic characteristics (mean age 66 years, standard deviation nine years, in both treatment groups; 67% and 68% females in EGb 761® and placebo group, respectively) and severity of BPSD (Table 1). Aberrant sleep/nighttime behavior (n = 1250), apathy (n = 1240), anxiety (n = 1231), irritability (n = 1168) and depression/dysphoria (n = 1015) were the most prevalent symptoms at baseline.
Table 1. Baseline composite and caregiver distress scores of the patients in the pooled data set; means ± standard deviations
Net changes between baseline and end of treatment for both composite and caregiver distress total scores were significantly larger in the patients treated with EGb 761® than in those receiving placebo. Regarding the single item composite scores, most pronounced net effects were found for apathy (LSMD −0.82; 95% CI −1.00 to −0.64), sleep/nighttime behavior (−0.64; −0.80 to −0.47), depression (−0.59; −0.72 to −0.46), anxiety (−0.58; −0.73 to −0.43), and irritability/lability (−0.48; −0.63 to −0.33), p < 0.001 for all in favor of EGb 761® compared to placebo (Figure 1). Similarly, net effects on the caregiver distress scale were largest for depression (LSMD −0.40; 95% CI −0.48 to −0.31), sleep/nighttime behavior (−0.38; −0.46 to −0.29), apathy (−0.37; −0.46 to −0.28), anxiety (−0.36; −0.45 to −0.27), and irritability/lability (−0.28; −0.37 to −0.19), p < 0.001 for all in favor of EGb 761® compared to placebo (Figure 2). There was little change in delusions, hallucinations, or elation/euphoria, the items with very low baseline scores (Figures 1 and 2). The I2 statistic, which quantifies the amount of variation in the results across the studies, ranged from 0% to 97% in the meta-analyses.
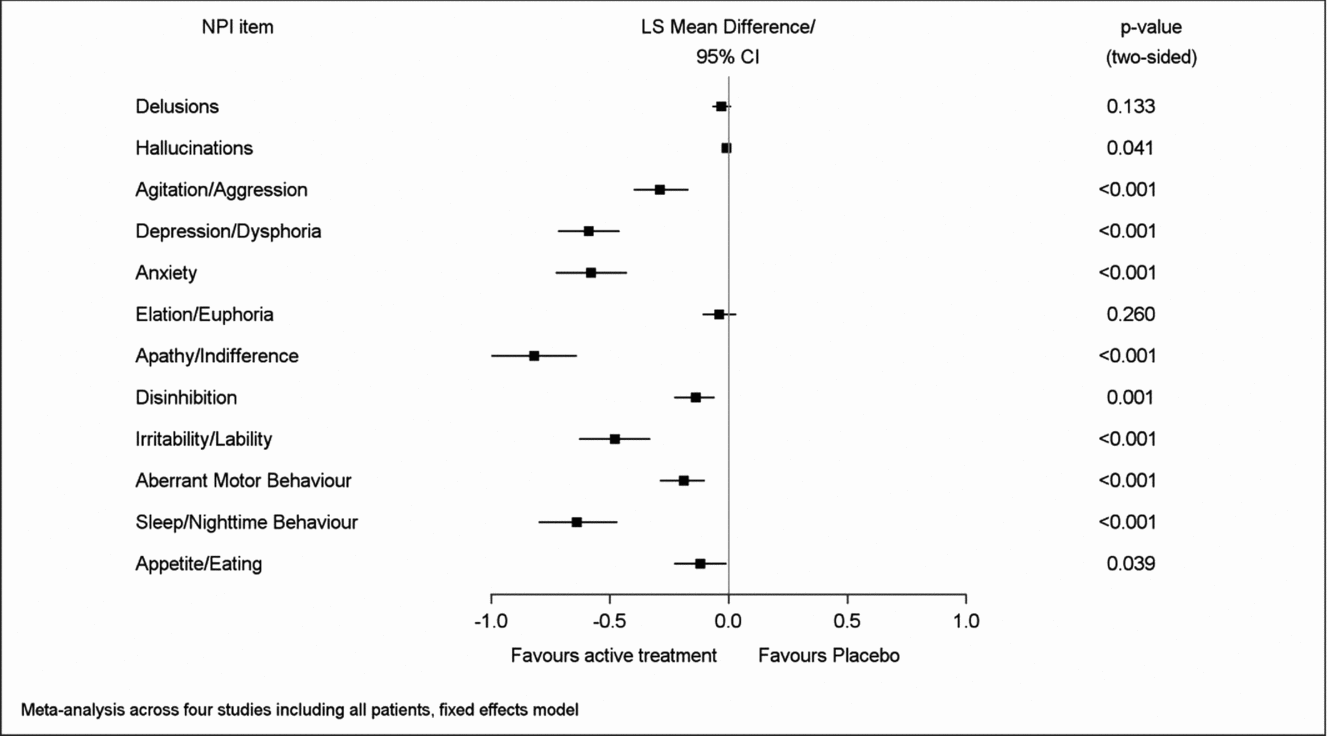
Figure 1. Overall effects of EGb 761® on symptom frequency/severity.
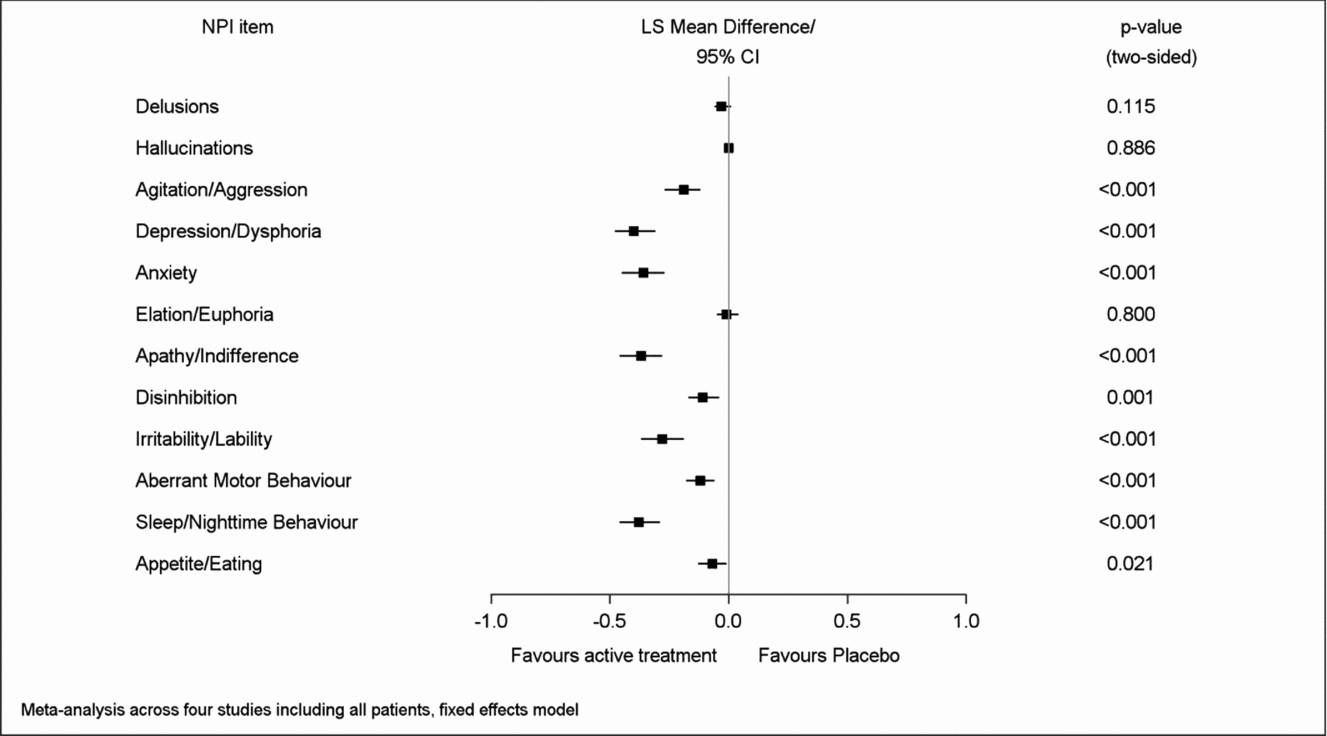
Figure 2. Overall effects of EGb 761® on caregiver distress.
Mean improvement of EGb 761® treatment compared to placebo for symptoms present at baseline (respective NPI item score > 0) was most pronounced in aberrant motor behavior (LSMD −1.05, 95% CI −1.43 to −0.67), apathy (−0.96; −1.17 to −0.74), depression (−0.83; −1.03 to −0.64), agitation (−0.82; −1.10 to −0.54), sleep/nighttime behavior (−0.78; −0.98 to −0.58), and anxiety (−0.74; −0.92 to −0.56), p < 0.001 for all in favor of EGb 761® compared to placebo (Figure 3). Caregiver distress was reduced most distinctly with regard to the same symptoms, although in slightly different sequence: agitation (LSMD −0.62; 95% CI −0.80 to −0.44), depression (−0.57; −0.69 to −0.44), aberrant motor behavior (−0.50; −0.70 to −0.30), sleep/nighttime behavior (−0.49; −0.59 to −0.38), anxiety (−0.48; −0.59 to −0.38), and apathy (−0.42; −0.53 to −0.31), p < 0.001 for all in favor of EGb 761® compared to placebo (Figure 4). Effect sizes (in favor of EGb 761® treatment) for these six items were classified as medium according to Cohen's concept, with SMDs between 0.44 and 0.59 in composite scores and caregiver distress scores.
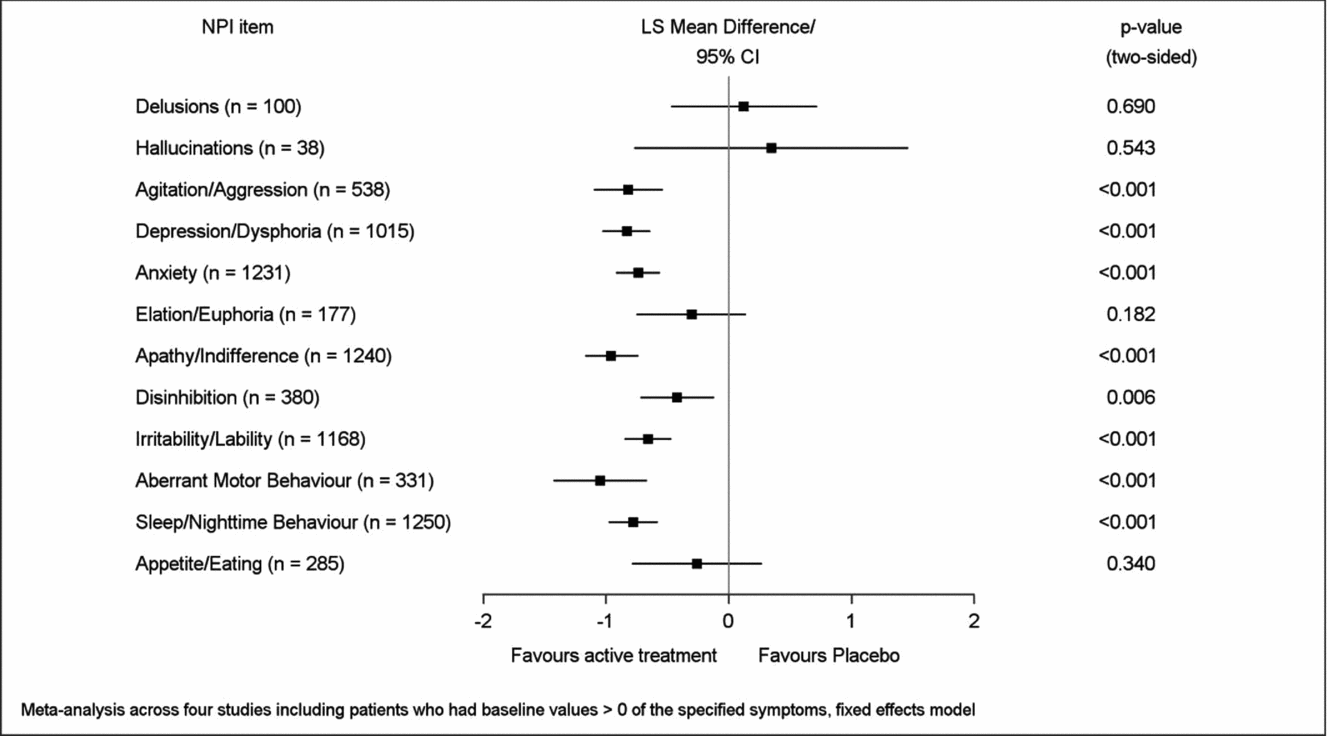
Figure 3. Improvement of symptom frequency/severity.
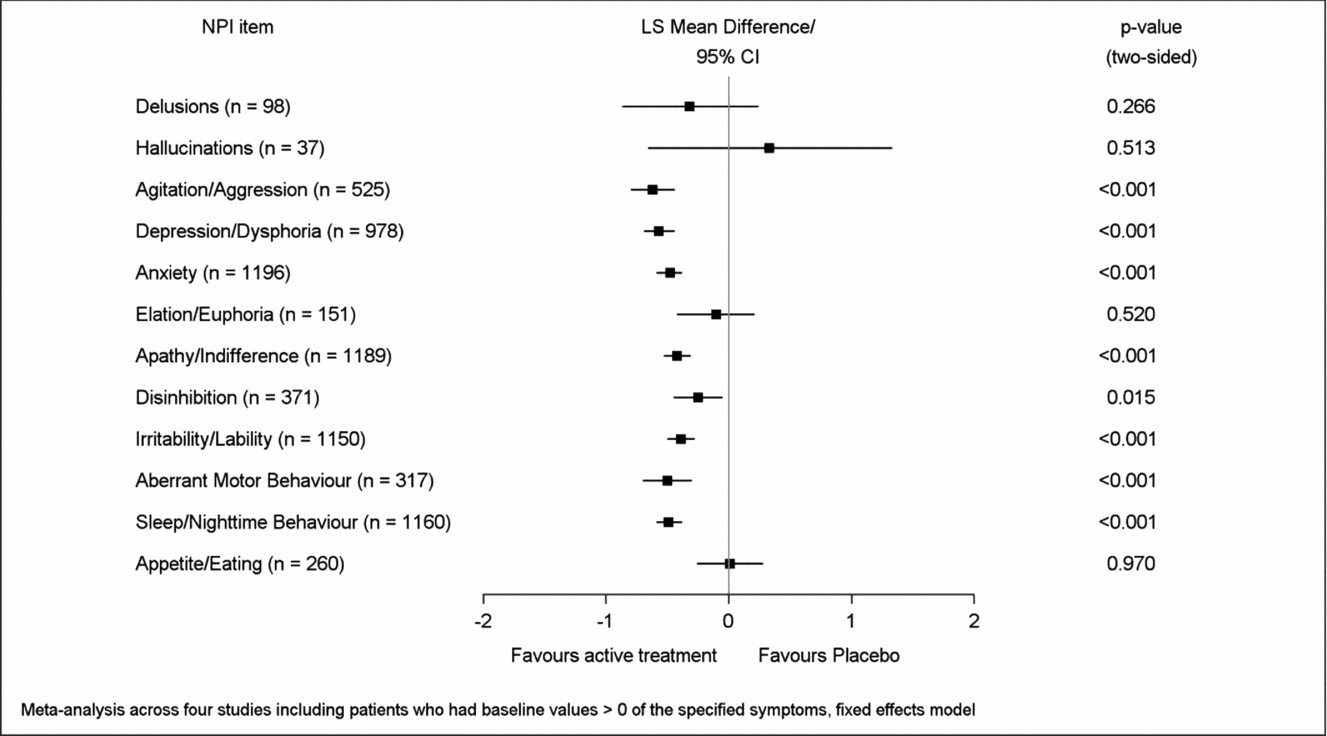
Figure 4. Improvement of caregiver distress.
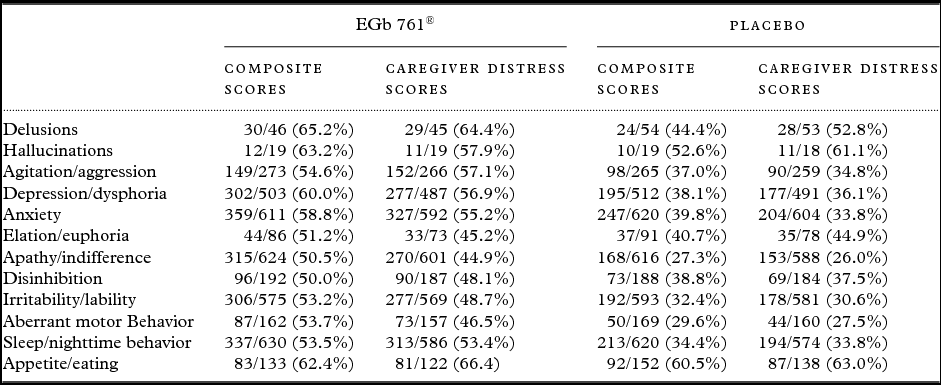
Improvements in symptoms that were present at baseline were more frequently observed in patients treated with EGb 761® than in those receiving placebo (Table 2).
Table 2. Numbers and percentages of patients who improved in symptoms that were present at baseline (number improved/number with symptom present at baseline (% improved))
The incidence of symptoms not present at baseline was significantly lower in EGb 761®-treated patients than in those receiving placebo for depression (risk ratio 0.36, (95% CI 0.22 to 0.60); p < 0.001), apathy (0.46 (0.28 to 0.75); p = 0.002), disinhibition (0.37 (0.21 to 0.67); p = 0.001) and disturbance of sleep/nighttime behavior (0.56 (0.34 to 0.93); p = 0.258). This was associated with lower rates of incident caregiver distress related to these symptoms. The risk ratios and 95% confidence intervals were 0.33 (0.20 to 0.55) for depression (p < 0.001), 0.63 (0.43 to 0.94) for apathy (p = 0.022), 0.43 (0.25 to 0.76) for disinhibition (p = 0.004), and 0.48 (0.30 to 0.79) for sleep/nighttime behavior (p = 0.004) in favor of EGb 761® compared to placebo.
Discussion
In four randomized, placebo-controlled trials, the efficacy of the quantified Ginkgo biloba extract EGb 761® was tested in patients who were suffering from dementia with clinically significant BPSD. Overall, in 9 out of 12 symptoms covered by the NPI significant benefits of EGb 761® in comparison to placebo could be demonstrated simultaneously for composite and caregiver distress over a period of 22 to 24 weeks. Although there was some heterogeneity with regard to effect sizes, the pattern of effects was remarkably consistent across the studies (cf. Scripnikov et al., Reference Scripnikov, Khomenko and Napryeyenko2007; Bachinskaya et al., Reference Bachinskaya, Hoerr and Ihl2011; Nacu and Hoerr, Reference Nacu and Hoerr2016).
To assess the clinical relevance of treatment effects, the same analyses were repeated in those patients in whom the respective symptoms were present at baseline. Composite score and caregiver distress score effect sizes were in the moderate range for apathy/indifference, disturbance of sleep/nighttime behavior, depression/dysphoria, anxiety, agitation, and aberrant motor behavior. For two symptoms that were less prevalent, agitation/aggression (n = 538) and aberrant motor behavior (n = 331), these analyses brought to light considerable effects on composite and caregiver distress scores, which were hidden in the overall analyses due to rather low average baseline values of these single items in the total population. For the symptoms that were most prevalent at baseline improvements were observed in 50% to 60% of the patients treated with EGb 761® and in 30% to 40% of those receiving placebo. Moreover, the risk of developing symptoms not present at baseline was reduced by more than 60% for depression and disinhibition, and approximately halved for apathy and disturbance of sleep/nighttime behavior in EGb 761®-treated patients compared to placebo-treated patients.
Our findings are in line with those reported from former studies of EGb 761® in dementia (summarized by Hoerr, Reference Hoerr2003) and vascular cognitive impairment (Halama et al., Reference Halama, Bartsch and Meng1988) that did not specifically enroll patients with BPSD, but accepted those with mild BPSD rather than excluding them. In these earlier studies, like in those reported here, depression, anxiety, irritability, emotional lability, and motivation were improved by EGb 761®, but the profile of effects was less pronounced. This may be due to the milder average severity of the disease and the different properties of the rating scales used. An earlier meta-analysis of EGb 761® trials found no significant effect on neuropsychiatric and behavioral symptoms overall, but the authors acknowledge that they included trials in “non-depressed and non-behaviorally disturbed patients” and that there were significant differences in favor of EGb 761® in the two studies included in the present meta-analysis and which enrolled patients with clinically significant BPSD (Weinmann et al., Reference Weinmann, Roll, Schwarzbach, Vauth and Willich2010).
The broad spectrum of effects of EGb 761® on BPSD probably reflects its complex pattern of pharmacodynamic activity, involving neuroprotection, enhancement of neurogenesis, and synaptogenesis, as well as modulation of neurotransmitter systems (Hoerr, Reference Hoerr2003; Tchantchou et al., Reference Tchantchou2009). The precise neurobiological mechanisms underlying each single BPSD remain to be elucidated. Basically, degeneration of neurons and synaptic connections result in a weakening of neuronal circuits and an imbalance of neurotransmitters, which entail both cognitive and emotional dysfunction (Geda et al., Reference Geda2013). Serotonergic effects of EGb 761® may play a role in the improvement of depression; inhibition of the stress axis may have an influence on anxiety (Hoerr, Reference Hoerr2003). Aberrant behaviors, such as agitation, aberrant motor behavior, and disturbed nighttime behavior may be due, to some extent, to cognitive impairment which results in misinterpretation of the environment and misunderstanding of other persons' behavior or verbal and facial expressions (Geda et al., Reference Geda2013). Improvement in cognitive domains involved in the recognition and interpretation of surroundings may therefore entail an improvement in aberrant behaviors.
One strength of the present analyses is the underlying (pooled) sample of approximately 1,600 patients with mild to moderate dementia who were selected using nearly identical eligibility criteria and who had NPI ratings at onset and end of 22- to 24-week treatment. Another strength of the data pool is that it comes from multi-center trials that involved sites in five European countries. It therefore seems reassuring that the profile of effects of EGb 761® is rather consistent across the studies.
One limitation of our study is the restriction to databases in English language when searching for relevant studies, although further studies may have been conducted in Asian countries. A search of Chinese and Japanese databases run by others for a different type of systematic review did, however, not bring to light any additional studies eligible for the present meta-analysis (Hyde et al., Reference Hyde2016). Another limitation is the relatively small number of patients with psychotic symptoms, i.e. delusions and hallucinations. This may be related to the fact that only outpatients with mild to moderate dementia were included in the studies and these symptoms are primarily observed in more severe forms of dementia. Although such symptoms may be very burdensome to caregivers, a treatment effect could not be shown. Elation is generally rare in patients with dementia, and so it was in the included trials.
Some improvement of BPSD has been reported for other anti-dementia drugs (Gauthier et al., Reference Gauthier, Wirth and Möbius2005; Rodda et al., Reference Rodda, Morgan and Walker2009), yet the overall effect sizes found in these meta-analyses appear smaller than those found for EGb 761®. However, in a study that directly compared EGb 761® and donepezil in patients with mild to moderate AD, essentially similar improvements were observed (Yancheva et al., Reference Yancheva, Ihl, Nikolova, Panayotov, Schlaefke and Hoerr2009). In the absence of direct comparisons with other drugs, any conclusion with regard to superiority would therefore seem premature.
In summary, analyses of pooled data from four large studies indicate that Ginkgo biloba extract EGb 761® is an effective treatment for BPSD in patients with mild to moderate dementia. Moderate benefits in a broad spectrum of symptoms, namely depression/dysphoria, agitation/aggression, aberrant motor behavior, apathy, sleep/nighttime behavior, anxiety, and irritability/lability were observed in more than 50% of patients over treatment periods of 22 to 24 weeks. In addition to improving the patients' symptoms, EGb 761® lowers the BPSD-related distress of their caregivers.
Conflict of interest
E. Savaskan, A. von Gunten, and S. Gauthier have not received any financial support for their participation in the present work; they have received speaker honoraria from Dr. Willmar Schwabe GmbH & Co. KG; Heiko Mueller and Robert Hoerr are full-time employees of Dr. Willmar Schwabe GmbH & Co. KG receiving fixed salaries.
Description of authors' roles
S. Gauthier and R. Hoerr developed the concept of the paper. H. Mueller performed the statistical analyses. All authors participated in the interpretation of the data and the writing of the paper. R. Hoerr and E. Savaskan coordinated the writing. All authors have approved the final paper.








