Published online by Cambridge University Press: 19 September 2011
At room temperature (diurnal range 23–28°C), Sarcophaga haemorrhoidalis (Fallen) developed from egg to adult in approx. 32 days. Survival (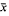 ± SE%) was generally high (76.63 ± 5.67 for the egg, 80.69 ± 3.20 for the larval and 89.83 ± 3.80 for the pupal stages). Adults emerged over a 4-day period between 07.00 and 13.00 hr local time, with daily peak emergence occurring between 07.00 and 10.00 hr. Their immediate post-emergence period was characterized by a highly active and mobile phase lasting 10–15 min followed by an immobile phase lasting about 1.5 hr. Copulation commenced 4.35 ± 0.43 days after adults emerged and was not preceded by elaborate courtship. After a pre-oviposition period of 12.96 ± 0.47 days, females deposited eggs in batches (1.57 ± 0.23 egg-batches/female) each containing a mean of about 37 ± 4 eggs. Fecundity was 52 ± 6 eggs/female during a mean adult life span of 18.68 ± 1.76 days. Although the foregoing life history parameters are strongly suggestive of K-selected ecological strategy, S. haemorrhoidalis may in fact be adopting an opportunistic r−selected ecological strategy since it habitually breeds in unstable environments.
± SE%) was generally high (76.63 ± 5.67 for the egg, 80.69 ± 3.20 for the larval and 89.83 ± 3.80 for the pupal stages). Adults emerged over a 4-day period between 07.00 and 13.00 hr local time, with daily peak emergence occurring between 07.00 and 10.00 hr. Their immediate post-emergence period was characterized by a highly active and mobile phase lasting 10–15 min followed by an immobile phase lasting about 1.5 hr. Copulation commenced 4.35 ± 0.43 days after adults emerged and was not preceded by elaborate courtship. After a pre-oviposition period of 12.96 ± 0.47 days, females deposited eggs in batches (1.57 ± 0.23 egg-batches/female) each containing a mean of about 37 ± 4 eggs. Fecundity was 52 ± 6 eggs/female during a mean adult life span of 18.68 ± 1.76 days. Although the foregoing life history parameters are strongly suggestive of K-selected ecological strategy, S. haemorrhoidalis may in fact be adopting an opportunistic r−selected ecological strategy since it habitually breeds in unstable environments.
Sarcophaga haemorrhoidalis (Fallen) s'est développé de l'oeuf à l'adulte en approximativement 32 jours, à une température d'intérieur. Le taux de survie (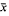 ± SE%) s'est révélé généralement élevé (76.63 ± 5.67 pour l'oeuf; 80.69 ± 3.20 pour le stade larvaire; 89.83 ± 3.80 pour la crysalide). Les adultes sortaient, au cours d'une période de quatre jours, entre 07.00 et 1300hr locales, la pointe journalière se situant entre 07.00 et 10.00 hr. La période qui suit directement la sortie des adultes se laisse classifier en deux phases: la première, d'un caractère très actif et mobile, dure de 10 à 15 min et est suivie de la deuxième, à caractère immobile, qui dure à peu près 1.5 hr. L'accouplement a commencé sans façons 4.35 ± 0.43 jours après la sortie des adultes. Après une période de pré-oviposition de 12.96 ± 0.47 jours, les femelles ont déposé des oeufs en grappes (1.57 ± 0.23 grappes d'oeufs la femelle) regroupant une moyenne de 37 ± 4 oeufs à peu près. La fécundité de chaque femelle s'est révélée de 52 ± 6 oeufs pour une vie adulte moyenne de 18.68 ± 1.76 jours.
± SE%) s'est révélé généralement élevé (76.63 ± 5.67 pour l'oeuf; 80.69 ± 3.20 pour le stade larvaire; 89.83 ± 3.80 pour la crysalide). Les adultes sortaient, au cours d'une période de quatre jours, entre 07.00 et 1300hr locales, la pointe journalière se situant entre 07.00 et 10.00 hr. La période qui suit directement la sortie des adultes se laisse classifier en deux phases: la première, d'un caractère très actif et mobile, dure de 10 à 15 min et est suivie de la deuxième, à caractère immobile, qui dure à peu près 1.5 hr. L'accouplement a commencé sans façons 4.35 ± 0.43 jours après la sortie des adultes. Après une période de pré-oviposition de 12.96 ± 0.47 jours, les femelles ont déposé des oeufs en grappes (1.57 ± 0.23 grappes d'oeufs la femelle) regroupant une moyenne de 37 ± 4 oeufs à peu près. La fécundité de chaque femelle s'est révélée de 52 ± 6 oeufs pour une vie adulte moyenne de 18.68 ± 1.76 jours.
Bien que les paramètres révélés laissent croire à une stratégie écologique de la K-sélection, il se peut que S. haemorrhoidalis suivent une stratégie écologique convenable de la r−sélection puisqu'il se reproduit par habitude dans des milieux instables.