Introduction
Acquired brain injury (ABI), such as stroke or traumatic brain injury (TBI), can lead to diverse consequences, ranging from physical disability and fatigue to cognitive impairments and behavioral changes (Reference Wilson, Winegardner, van Heugten and Ownsworth1;Reference Teasdale and Engberg2). As a result, people with ABI often need to cope with a new reality and way of life. People can experience loss regarding independence, occupation, social roles, and social contacts. Following an ABI, around 20–40 percent of individuals experience anxiety and/or depression, often co-occurring (Reference Rapoport3–Reference Rafsten, Danielsson and Sunnerhagen9).
Although treating these symptoms remains a challenge, evidence suggests that Acceptance and Commitment Therapy (ACT) can be an effective treatment for ABI-related anxiety and depressive symptoms (Reference Whiting, Deane, McLeod, Ciarrochi and Simpson10–Reference Rauwenhoff, Bol, Peeters, van den Hout, Geusgens and van Heugten13). Meta-analyses also show that ACT can be an effective treatment for anxiety and depressive symptoms in people without ABI, addiction, obsessive-compulsive disorder, and schizophrenia (Reference JG, Davis, Morina, Powers, Smits and Emmelkamp14–Reference Gloster, Walder, Levin, Twohig and Karekla16). ACT, a third-wave behavioral therapy that aims to improve psychological flexibility, which is defined as paying attention to the present moment with (negative) thoughts and feelings while basing your behavior on the context and personal values (Reference Hayes, Luoma, Bond, Masuda and Lillis17). Therefore, an important part of ACT is value exploration where people identify their most important values (i.e., family, nature, contributing to society). An important therapy outcome is the increase of value-driven behavior, which is behavior based on these personal values (Reference Hayes, Luoma, Bond, Masuda and Lillis17). Furthermore, compared to other psychotherapies, such as cognitive behavioral therapy, thoughts are not altered or changed during ACT, but rather patients learn not to fight against their thoughts and feelings and accept them. This is likely a fitting approach for people with chronic health conditions as their negative thoughts and feelings can be quite realistic given their situation (i.e., anxiety about a stroke recurrence). Recently, BrainACT was developed, an adapted form of ACT specifically designed for individuals with ABI. It addresses their unique needs and cognitive and communication challenges, ensuring accessible and tailored therapy (Reference Rauwenhoff, Bol, van Heugten, Batink, Geusgens and van den Hout18). BrainACT has been shown feasible (Reference Rauwenhoff, Bol, Peeters and van Heugten19) and promising in terms of effectiveness (Reference Rauwenhoff, Bol, Peeters, van den Hout, Geusgens and van Heugten13).
There is evidence that ACT is cost-effective over comparators for several other patient populations (Reference Risør, Frydendal, Villemoes, Nielsen, Rask and Frostholm20–Reference Duarte, Lloyd, Kotas, Andronis and White23). However, no prior research has been conducted on the cost-effectiveness of BrainACT. This article investigated BrainACT’s cost-effectiveness and cost-utility from a societal perspective for people with anxiety and/or depressive symptoms following ABI compared to an active control arm (psychoeducation and relaxation intervention) over a 1-year time horizon in the Netherlands.
Methods
An economic evaluation was conducted as part of the BrainACT study (see Reference (Reference Rauwenhoff, Peeters, Bol and Van Heugten24) for the trial protocol). The design of the study was a multicenter randomized controlled two-armed parallel trial. Stakeholders were not involved in the design, approach, and results of the study. The economic evaluation applied a societal perspective, which is the recommended perspective in the Dutch guidelines for economic evaluations (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25). The sample size was powered for the clinical outcomes and calculated for 94 participants (80 excluding anticipated dropouts). A 1-year time horizon was adopted as it matched the follow-up period of the study. A full health-economic analysis plan was not developed before the analysis. Ethical approval for the study has been given by the medical research ethics committee of Maastricht University Medical Centre and Maastricht University and the local committees of participating clinical centers (reference number: NL65349.068.18). The study was registered in the Dutch Trial Register (now Clinical Trial Registry Platform) (reference numbers: NL691 and NTR 7111). This article follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS 2022) guideline (Reference Husereau, Drummond, Augustovski, de Bekker-Grob, Briggs and Carswell26) (see Supplementary File 1).
Study population
The target groups were people, aged 18 years and above, who sustained an objectified TBI or stroke and experienced depressive and/or anxiety symptoms as measured by the Hospital Anxiety and Depression Scale (HADS) (Reference Zigmond and Snaith27). The full eligibility criteria are described in Supplementary File 2 or found in the protocol paper (Reference Rauwenhoff, Peeters, Bol and Van Heugten24).
Procedure
Participants from Dutch healthcare facilities (hospitals, rehabilitation centers, and mental healthcare facilities) were recruited between April 2019 and January 2022. Potential participants were referred to a psychologist, screened for eligibility, and informed about the study. If eligible participants provided informed consent, the initial measurement (T0) was conducted. Hereafter, participants were randomly allocated to the BrainACT or the active control arm by an independent third party using computerized block randomization (block 6, 1:1 allocation). Economic evaluation data were collected at 3 months (T1 – postintervention), 7 months (T2), and 12 months (T3) follow-up. Data entry, review, and verification were performed by blinded research assistants.
Intervention and comparator
The ACT intervention involved eight individual sessions of 1 hour for 3 months. Participants did homework exercises of around 30 minutes for 6 days per therapy a week, consisting of reading or listening to summaries of the sessions and practicing ACT skills. The BrainACT intervention was administered individually by psychologists who completed an ACT training program of at least 5 days and were experienced in treating patients with ABI. The existing ACT protocols were adapted to fit the needs of people with ABI. A detailed description and rationale of the BrainACT intervention can be found elsewhere (Reference Rauwenhoff, Bol, van Heugten, Batink, Geusgens and van den Hout18).
The active control intervention consisted of an eight-session psychoeducation intervention combined with relaxation training with a duration of 1 hour. This comparator was chosen as psychoeducation is frequently utilized and is a well-recognized treatment for ABI in Dutch clinical practice (Reference Lafosse28). The psychoeducation is based on “Niet rennen maar plannen” (do not run, plan) (Reference Donnellan, Hickey, Hevey and O’Neill29), which is a training and educational program focusing on cognitive rehabilitation for patients with ABI and mild cognitive problems. The relaxation training consisted of a muscle relaxation (Reference Wilz30) and an autogenic training (Reference Chamelian and Feinstein31). Participants did homework exercises of around 30 minutes for 6 days per therapy week. The intervention was provided individually by a healthcare professional with experience in working with ABI.
Treatment protocols were specified for both intervention and comparator to ensure comparability of the education interventions across settings. All care professionals received a short training provided by the researchers before the study on providing their respective interventions. Both interventions took place at hospital outpatient facilities and are explained more in detail elsewhere (Reference Rauwenhoff, Peeters, Bol and Van Heugten24). Participants received usual care except for unstable psychotropic medication or previously received ACT were excluded (see Supplementary File 2).
Valuation of outcomes
The cost-effectiveness analysis (CEA) health outcome was anxiety and depression using the Hospital and Anxiety Depression Scale (HADS). The HADS (Reference Zigmond and Snaith27) provides separate scores for depression and anxiety domains, each with seven items self-rated on a 4-point Likert scale ranging from zero (not at all) to three (a great deal of the time). The total scores range from 0 to 21 per domain, with higher scores indicating higher anxiety or depression levels. Good reliability was detected in previous research (Reference Bjelland, Dahl, Haug and Neckelmann32) and for people with ABI (Reference Whelan-Goodinson, Ponsford and Schönberger33).
The cost-utility analysis (CUA) outcome was health-related quality of life and was measured using the five-dimensional five-level EuroQol (EQ-5D-5L) instrument. The EQ-5D-5L (Reference Herdman, Gudex, Lloyd, Janssen, Kind and Parkin34) measures health-related quality of life covering mobility, self-care, daily activities, pain/discomfort, and anxiety/depression. Each dimension is rated on a 5-point Likert scale ranging from “no problem” to “unable to do.” This scale is the recommended generic preference-based scale for health-economic analysis in the Netherlands (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25). The Dutch utility tariff of the EQ-5D-5L was used to estimate the utility of the participants’ health states (Reference Versteegh and Brouwer35). Utility reflects the health state of participants on a scale from one (perfect health) to zero (death). A utility below zero was also possible, indicating a health state worse than death. Total quality-adjusted life-years (QALYs) were calculated by multiplying the utilities of the health states by the time between two measurement moments and summed over the 12-month follow-up period using the area under the curve approach.
Valuation of resources and costs
The resource usage of the participants was measured at each timepoint with a self-report 15-item cost questionnaire, which was constructed to collect cost data from a societal perspective based on the steps described by Thorn et al. (Reference Thorn, Coast, Cohen, Hollingworth, Knapp and Noble36). The cost questionnaire used in this study was based on a questionnaire used in the study of Kootker et al. (Reference Kootker, Rasquin, Lem, van Heugten, Fasotti and Geurts37) and adopted a fixed recall period of 3 months. Inconsistencies or missing data in the collected resource use data were discussed between authors SLO and JR to handle or correct the data. Three main cost categories were adopted and calculated in Euros (€): (1) intervention, (2) healthcare, and (3) non-healthcare costs.
Intervention costs were calculated per participant by multiplying the number of sessions in the BrainACT or active control intervention by the unit price of the corresponding type of care professional that delivered the sessions. Training costs were not included in the intervention cost calculation, considering the participating care professionals were already certified ACT-therapists. Healthcare costs were calculated by multiplying the resource usage by its corresponding unit price, over the 12-month follow-up period. The unit prices in euro (€) were adopted from the Dutch guidelines (2014) (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25) and inflation-adjusted (factor of 1.22) to 2022 using the Dutch consumer price index (38). Costs were obtained over a fixed recall period (3 months) and were extrapolated to the full period between the observations (4 months for T2 and 5 months for T3) (see Supplementary Figure 1 for a schematic overview of these cost corrections). Finally, non-healthcare costs, including unpaid help, paid, and unpaid productivity losses were calculated. The friction cost approach was used to estimate the lost productivity costs, using the measurements of productivity loss as obtained by the cost questionnaire. The friction period was calculated at 138 days based on the Dutch guidelines (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25) and vacancies 2022 data from CBS [Statistics Netherlands] (39). One day of (un)paid productivity loss was assumed to equal 7.6 hours, the assumed average hours of a working day (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25). Informal care hours were valued based on replacement costs for the standard hourly rate of home help, as recommended by the Dutch costing guidelines (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25). Discounting was not applied, as the trial follow-up period did not exceed 1 year. Supplementary Table 1 provides an overview of all the included cost categories with their corresponding unit prices and units of measurement.
Analytical methods
All statistical analyses were performed using STATA (version 17.0, Standard Edition). All data were analyzed according to the intention-to-treat principle. Missing data were handled following the guidance described in Faria et al., (Reference Faria, Gomes, Epstein and White40). Missing values were tested for missingness and imputed using multiple imputations at the endpoint level. For further details on handling missing data and the STATA code of the multiple imputation model, please refer to Supplementary File 3.
In the base-case analysis, both the incremental cost-effectiveness ratio (ICER) and the incremental cost-utility ratio (ICUR) were calculated for the CEA and CUA, respectively. The ICER was calculated by dividing the incremental costs by the incremental effects (total HADS score) between both arms. The ICUR was estimated by dividing the incremental societal costs by the incremental QALYs. Incremental outcomes (total costs, QALYs, and HADS) were estimated using a mixed model with covariates baseline status on the outcome, arm, and centrum as a random intercept. The significance of the coefficient “arm” represented the adjusted difference between the two arms and was tested for significance. To handle uncertainty, bootstrapping with 1,000 replications was used to estimate 95 percent bootstrap intervals around cost, QALY, and effect differences. Bootstrapped differences in outcomes, cost-effectiveness, and cost-utility were visualized in incremental cost-effectiveness and cost-utility planes. The cost-effectiveness probability was then displayed at a willingness-to-pay threshold, representing the amount society is willing to pay to gain one QALY. This threshold varies per country. In the Netherlands, the Care Institute recommends different thresholds based on the disease burden severity (Reference Zwaap, Knies, van der Meijden, Staal and van der Heiden41). The disease burden of the study’s population was assumed as moderate by the researchers; therefore, the €50,000/QALY threshold was adopted.
Scenario and subgroup analyses
Six scenario analyses (SAs) were conducted to test the robustness of the findings. First (SA1), a healthcare perspective was employed, excluding non-healthcare costs (unpaid help and (un)paid productivity losses). Although the societal perspective is recommended (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25), the healthcare perspective offers another alternative viewpoint for policymakers. In the second analysis (SA2), the human capital approach was used instead of the friction cost method to estimate productivity costs. Inconsistencies and unrealistic values were detected for the productivity loss data for 9 out of the 72 participants (12.5 percent). In the base case, these concerning data were corrected or handled in consensus between authors SLO and JR. However, the third SA entailed a more stringent approach where these records were excluded from the analysis (SA3). For SA4 the U.K. value set of the EQ-5D-5L utilities (Reference Devlin, Shah, Feng, Mulhern and van Hout42) was used as an alternative to the Dutch value set. The fifth analysis (SA5) examined the impact of handling missing data through list-wise deletion, an alternative to the multiple imputation model used in the base case.
The robustness of the outcomes was further investigated in three subgroup analyses (SGAs) as the researchers expected these groups might have different treatment responses and therefore, potentially influence anxiety and/or depressive symptoms, quality of life, or care use.” The groups were age (≥60/<60 years), gender (male/female), and employment status (working/not working at baseline).
Results
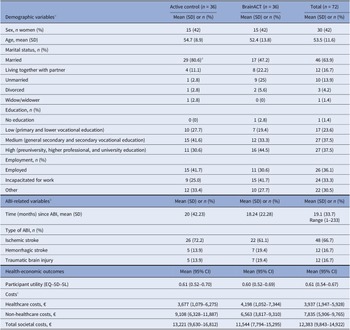
A total of 72 stroke and TBI survivors were included in this study, and all were included in the analysis due to the intention-to-treat principle. Participants were recruited from 12 different sites. Among them, 50 individuals (69 percent) were recruited from seven hospitals, 21 participants (29 percent) from four rehabilitation centers, and one participant (1 percent) from a mental healthcare facility. Supplementary Figure 2 shows the study’s flowchart. Half of the participants (n = 36) were randomized to the active control arm and the other half (n = 36) to the BrainACT arm. The mean age of the participants was 54 years (SD of 11.6), and 30 (42 percent) of them were females. At the start of the study, 58 (81 percent) of the participants were married or living with a partner, 27 (38 percent) had followed higher education, and 26 (36 percent) were employed. For details on demographic baseline characteristics, see Table 1.
Table 1. Baseline characteristics of the 72 patients included in this study

Abbreviations: ABI = acquired brain injury, SD = standard deviation, and 95% CI = 95% confidence interval.
a A significant difference (<0.05) was detected between arms at baseline using chi-square test.
b Copied from Rauwenhoff, J. Bol, Y. Peeters, F., Smits, P., Duits, A., Wijenberg, M., Blok A., van Heugten, M. (2024). Acceptance and Commitment Therapy for people with depressive and anxiety symptoms following acquired brain injury: results of the BrainACT randomized controlled trial, with permission from authors.
c Collected with a recall period of 3 months.
Missing data
At baseline, there were no missing data. During the follow-up period, a total of 13 (18 percent) participants dropped out (n = 9 from the active control arm and n = 4 from the BrainACT arm). Of the 288 targeted observations, the resource usage and EQ-5D-5L questionnaires were completed in 82 percent (n = 237) of the cases. Of all participants (partly) missing observations occurred due to drop-out 13 percent (n = 36), due to full missing visits 3 percent (n = 10), and due to item-level missing 2 percent (n = 5) (i.e., one item of the EQ-5D-5L was missing but the other four items were complete for one participant at T1) (see Supplementary Table 2 for an overview of the endpoint missingness at baseline and follow-up). The missingness status on utility, total costs, and HADS at each observation was not significantly predicted by baseline or its previous observation demographic, utility, costs, or HADS. Therefore, missingness was assumed missing-completely-at-random.
Costs
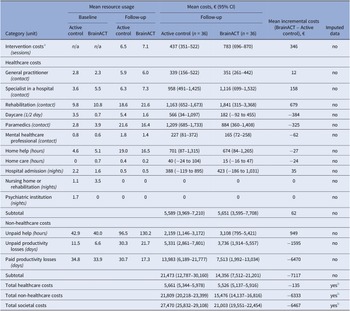
The mean intervention costs were higher in the BrainACT arm (€783) compared to the active control arm (€437). For a detailed overview of the intervention cost calculation, see Supplementary File 4. The total imputed healthcare costs were €5,661 for the active control arm and €5,526 for the BrainACT arm. However, non-healthcare costs were larger for the active control arm (€21,809) compared to the BrainACT arm (€15,476). Paid productivity losses showed the largest mean difference, with an incremental difference of €6,470 between arms. Considering all accumulated cost categories during the 12-month follow-up period, the active control arm had higher total mean societal costs (€27,470) compared to the BrainACT arm (€21,003). See Table 2 for an overview of all costs during the trial period.
Table 2. Resource and costs (in Euros (2022)) of the non-imputed individual cost items and the imputed total cost categories at baseline and 12-month follow-up (n = 72)

Abbreviations: 95% CI = 95% confidence interval.
a See Supplementary File 4 for a detailed overview of the intervention cost calculations.
b Imputation was done on the endpoint level. Therefore, only the total cost categories have imputed data available.
Outcomes
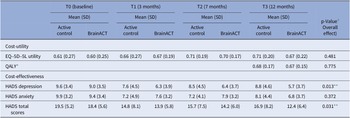
The results from Table 3 indicate the EQ-5D-5L-based utility scores and HADS (anxiety, depression, and total) scores for both study arms over the 12-month follow-up period. The difference between arms was statistically tested using a mixed model including the interaction between time and arm (see Table 3 for details). Throughout this period, both arms had a utility increase, but it did not differ significantly between arms (p = 0.481). In addition, QALYs (p = 0.775) and HADS anxiety scores (p = 0.372) did not show significant differences between arms. However, the active control arm showed significantly higher HADS depression scores (p = 0.013) and HADS total scores (p = 0.031), indicating increased anxiety/depression compared to BrainACT.
Table 3. Average imputed outcomes for utility, QALY, and depression and anxiety scores (n = 72)
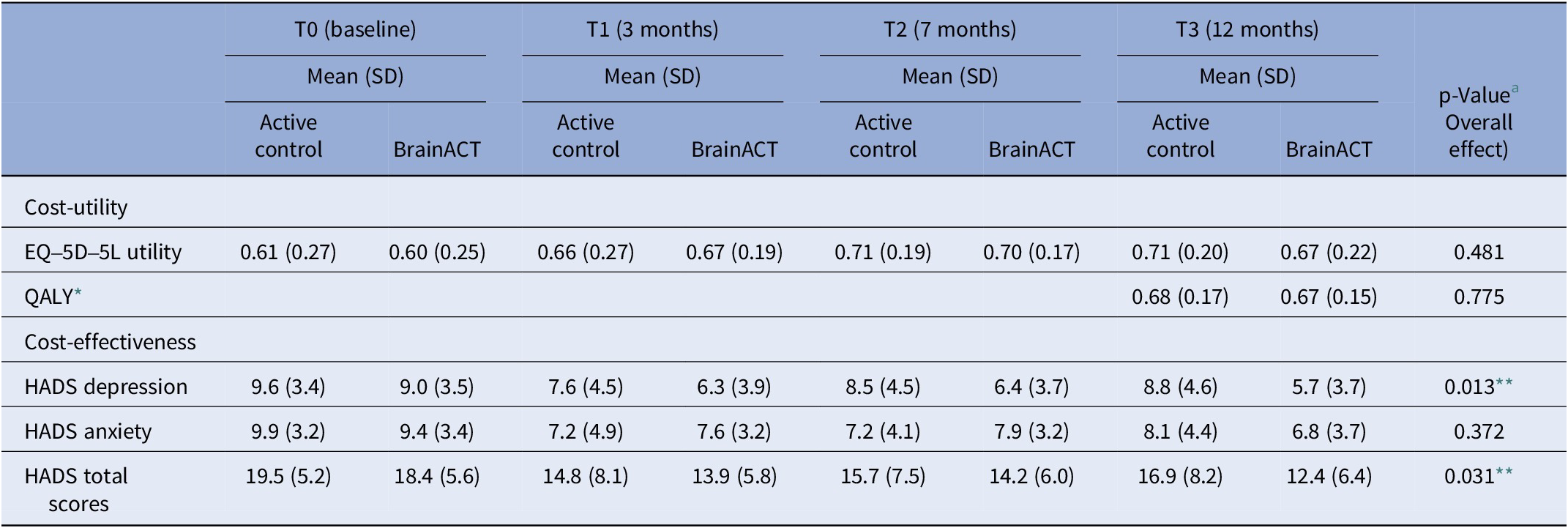
Abbreviations: SD = standard deviation, QALY = quality-adjusted life-year, and HADS = Hospital and Anxiety Depression Scale.
* QALY calculated for the follow-up period (0–12 months).
** Statistically significant difference (p < 0.05).
a Linear mixed model includes all measurement moments: independent variables are condition, time (continuous), the interaction between time and condition, random intercept expressed at the center and participant level (observations nested within participants and participants nested within centers); the dependent variable contains observations from baseline, T1, T2, and T3. P-value corresponds to the parameter “interaction between time and condition.”
Base-case analysis
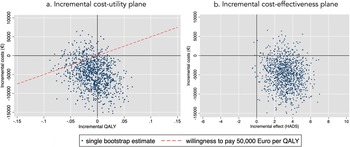
After bootstrapping, the CUA from a societal perspective showed that BrainACT participants achieved fewer mean QALYs (−0.008; bootstrap interval −0.060 to 0.042) and accrued lower mean costs (€−4,881; bootstrap interval €−12,139 to €2,330) compared to active control participants. Because these incremental QALYs and incremental costs were both negative, the results presented an ICUR of €610,125 savings per QALY loss. The incremental cost-utility plane (see Figure 1a) showed that 54 percent of the bootstrapped ICURs were located in the southwest quadrant (less costs and less QALY) and 37 percent in the southeast quadrant (less costs and more QALY). The cost-utility acceptability curve (see Supplementary Figure S3) revealed that the probability of BrainACT being cost-effective was 86 percent at a willingness-to-pay (in this case willingness-to-accept a saving per QALY loss) level of €50,000/QALY.

Figure 1. (a) Incremental cost-utility plane. (b) Incremental cost-effectiveness plane.
The CEA from a societal perspective revealed that the BrainACT arm was dominant, with lower mean total costs (€−4,881) along with greater effects in terms of HADS (3.2; bootstrap intervals 0.7–5.7). Consequently, the ICER amounted to €−1,525 savings per gained point improvement on the HADS. The incremental cost-effectiveness plane (see Figure 1B) showed that the majority of bootstrapped ICERs (90 percent) resided in the dominant southwest quadrant (lower costs and higher effects).
Scenario and subgroup analyses results
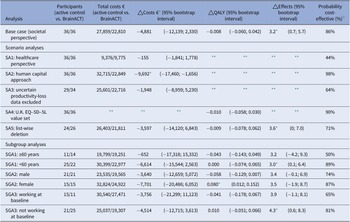
The SA from a healthcare perspective (SA1) resulted in lower incremental costs than the base case (−€155 vs. −€4,881) and a lower cost-effectiveness probability (44 percent compared to the base case’s 86 percent probability). SA2, where the human capital approach was adopted, showed significantly higher incremental costs (−€9,692) and cost-effectiveness probability (98 percent). For the SA where all records with uncertainty surrounding the productivity losses were excluded (SA3), showed lower incremental costs (−€1,948), and the chance of BrainACT being cost-effective was lower (64 percent). The outcomes for SA4, where the U.K. utility value set was adopted, and SA5, where list-wise deletion was used to handle missing data, remained robust.
For subgroup 1, the incremental costs were lower for individuals who are 60 years old or older compared to participants aged below 60 years old (−€652 vs. -€6,614). This resulted in a higher cost-effectiveness probability for participants under 60 years old (89 percent vs. 50 percent). Females experienced a significant positive increase in QALYs (0.080), whereas males experienced QALY decreases (−0.058). The outcomes for SGA3 (working vs. not working) remained robust. For further details on the SAs and SGAs, see Table 4.
Table 4. Bootstrapped results (bootstrap mean and 95% bootstrap interval) of the base case, scenario analyses, and subgroup analyses

Abbreviations: QALY = quality-adjusted life-year, SA = scenario analysis, UK = United Kingdom, SGA = subgroup analysis.
* Significant difference at the 0.05 level based on the 95% bootstrap intervals.
** Same as the base case.
a The incremental costs were based on mixed model which includes correction for baseline. Therefore, they vary from the total cost difference between arms.
b At a willingness-to-pay thresholds for a moderate disease burden of €50,000/QALY.
Discussion
This study demonstrated that BrainACT led to lower total costs and decreased anxiety, both non-significantly, while significantly reducing depression. Therefore, BrainACT dominated the active control in the CEA. In the CUA, a minor, non-significant decrease in health-related quality of life was observed. However, the cost savings resulted in a high probability of being cost-effective from a societal perspective, if willing to accept the minor QALY loss.
The non-significantly lower total costs in the BrainACT arm were mainly driven by productivity costs. Missed working days were reduced in both arms, but more noticeable in the BrainACT arm. Possibly because ACT aims to increase value-driven behavior (Reference Hayes, Luoma, Bond, Masuda and Lillis17) and labor might be considered by many as an important value in life. The results were sensitive to the method of valuing productivity. Except when productivity loss was fully omitted, BrainACT was cost-effective in all scenarios.
Although both anxiety and depression decreased in the BrainACT arm, only depression reduction was statistically significant. However, the study’s associated clinical effectiveness analysis showed that more participants in the BrainACT arm clinically significantly improved on both outcomes (defined as the patient recovered and significantly improved) (Reference Rauwenhoff, Bol, Peeters, Smits, Duits and Wijenberg43).
To the best of the authors’ knowledge, this is the first health-economic evaluation on ACT for people with anxiety and/or depressive symptoms following ABI, with no similar studies to compare. Nevertheless, our results were in line with a Danish trial-based economic evaluation investigating online ACT for patients with severe health anxiety (Reference Risør, Frydendal, Villemoes, Nielsen, Rask and Frostholm20). In this study, ACT dominated the active control condition from a societal perspective. Mean sick leave decreased from 2.4 weeks at baseline to 0.6 after 6 months, whereas it increased for the active control arm from 1.0 to 1.1 weeks.
The costs of BrainACT (€783) were higher than a psychoeducation-based intervention combined with relaxation exercises (€437). Despite having a similar number of sessions and intensity, BrainACT was only delivered by psychologists while the active control intervention was delivered by psychologists and other healthcare professionals, who on average had lower employment costs.
Strength and limitations
This study’s strengths include recruitment from diverse Dutch sites and healthcare facilities, enhancing generalizability; high intervention fidelity with 75 percent protocol compliance and 98 percent overall session attendance 98 percent (Reference Rauwenhoff, Bol, Peeters and van Heugten19); and analysis adherence to the Dutch economic evaluations guidelines (Reference Hakkaart, Nvd, Bouwmans, Kanters and Tan25).
The study also has some limitations. First, the sample size (n = 72) was lower than its target (n = 94), which may have reduced the statistical power. This made it harder to determine whether the observed differences were caused by the intervention or chance. Therefore, the results should be interpreted cautiously due to the potential impact on reliability and validity. In addition, the SGAs were limited in interpreting due to the small sample size. Second, productivity data contained inconsistencies (i.e., productivity days exceeding the observation interval), possibly due to differences in the interpretation of the research protocol by those collecting the data, which were solved by truncation. However, the results were still robust when these inconsistent records were excluded (SA3). Third, follow-up was only 1 year, and this time frame might be too short to adequately evaluate the cost-effectiveness of an intervention, such as BrainACT, where mainly long-term effects were anticipated.
Finally, only the consultation costs were included in the intervention costs. Other potentially relevant costs, such as material and facility costs, were not included due to limited information available in the research database. However, we estimated that these costs would be similar for both interventions and therefore have a small effect on the trial results. This study’s negative ICERs are ambiguous to interpret and therefore not ideal to present (Reference Shields and Elvidge44, Reference Paulden45). To avoid misinterpretations or spurious conclusions based on the ICER/ICUR, we focused more on presenting and discussing the incremental outcomes and cost-effectiveness probability instead of the ratios in this article.
Considering the study’s limitations, future research should aim to obtain more robust evidence on BrainACT by addressing these limitations. Priorities include increasing the sample size, extending the follow-up period, and refining data collection protocols, particularly for productivity-related data, to enhance consistency and reliability.
Clinical implications
Despite the uncertainty arising from the trial’s limitations, we deem the results sufficiently reliable to recommend considering the implementation of BrainACT for people with anxiety and/or depressive complaints following ABI in the Netherlands, as it is likely more cost-effective than a psychoeducation and relaxation intervention. In addition, ACT is an accessible therapy, requiring no extensive certification process for care professionals (46), facilitating implementation. Furthermore, BrainACT demonstrated the potential for improving productivity, which we believe should be highly valued in an aging society to support the availability of labor.
The trial demonstrated increased utility and reduced anxiety/depressive symptoms in both intervention arms. Therefore, policymakers could also consider BrainACT to complement the existing intervention, with the choice depending on patient preferences, to promote personalized ABI treatment. However, due to the presented uncertainty, we would recommend monitoring the effect of BrainACT (e.g., on productivity losses) during its implementation to further confirm the trial’s results.
Conclusion
Results of this trial-based health-economic evaluation show good promise for BrainACT being a more cost-effective alternative to psychoeducation and relaxation interventions for people with anxiety and/or depressive complaints following ABI, as observed over a 1-year follow-up period from a societal perspective. However, the findings are constrained by limitations. Despite this, BrainACT shows potential as a valuable addition to current treatment options. Future research should confirm these results with more robust evidence. Meanwhile, implementing BrainACT in clinical settings accompanied by continued monitoring could be considered.
List of abbreviation
- ABI
-
acquired brain injury
- ACT
-
Acceptance and Commitment Therapy
- CEA
-
cost-effectiveness analysis
- CUA
-
cost-utility analysis
- HADS
-
Hospital and Anxiety Depression Scale
- ICER
-
incremental cost-effectiveness ratio
- ICUR
-
incremental cost-utility ratio
- QALYs
-
quality-adjusted life-years
- SA
-
scenario analysis
- SGA
-
subgroup analysis
- TBI
-
traumatic brain injury
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0266462324004811.
Data availability statement
The data supporting the findings of this study are available upon reasonable request from the corresponding author.
Acknowledgments
The authors would like to thank the participants and therapists for participating in this study. The authors would also like to thank the participating centers: Adelante, Hoensbroek; Catharina Ziekenhuis, Eindhoven; Deventer Ziekenhuis, Deventer; Geestelijke Gezondheidszorg Eindhoven, Eindhoven; Medisch Centrum Leeuwarden, Leeuwarden; Maastricht Universitair Medisch Centrum+, Maastricht; Noordwest Ziekenhuisgroep, Alkmaar; Revalidatie Friesland, Leeuwarden; Saxenburgh Ziekenhuisgroep, Hardenberg; Sint Maartenskliniek, Nijmegen; UMCG Centrum voor Revalidatie Beatrixoord, Haren; Zuyderland Medisch Centrum, Heerlen en Sittard/Geleen. Furthermore, the authors would like to thank Roos Roberts, Jeroen Woudstra, Andreïna Martina, Rianne Hazenberg, Gea Klein, Anaïs Nkenda, Twan Roex, Anouk Hollands, Dana Hermsen, and Ilse Keijdener for the help conducting the measurements.
Authors contribution
Funding was obtained by CvH. The concept and design of the study were drafted by CvH, SMAAE, and JR. Data were collected by JR and CvH. The statistical analysis was executed by SLO and RH. The manuscript was drafted by SLO and JR. The authors involved in the analysis and interpretation of data were RH, SLO, GAPGvM, and JR. All authors critically reviewed and approved the final manuscript. Sander Osstyn and Johanne Rauwenhoff shared first author.
Funding statement
The study was funded by ZonMW (grant No. 636310003). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the health-economic evaluation paper.
Competing interest
The author(s) declare none. Outside of this study, author R.H. received consulting fees in the past 3 years from Lilly Nederland (2023), iMTA (2023), and Biogen (2021) (paid to institution).