Background
Highly innovative technologies (such as immuno-, cell, and gene therapies that use novel molecular biology to target the underlying cause of a disease) aim to deliver transformative patient benefit. Where large potential benefit is expected in areas of high unmet medical need (such as in rare diseases), regulators have developed expedited pathways. Regulatory approval based on interim analyses of short-term end points and early data cut-offs is common, as is the use of uncontrolled trials. This means that some health technologies enter the market with a limited evidence base to demonstrate clinical effectiveness.
There is substantial pressure for Payers (or health technology assessment [HTA] bodies) to make (or inform) pricing and reimbursement decisions about these technologies at the point of market launch. However, uncertainties exist about the population to be treated, natural history of disease, size, and durability of clinical effects compared to treatment alternatives, safety, cost effectiveness, and budget impact. As, these highly innovative technologies often have a high price due to the complexity of development and purported high patient benefit, decisions about their value are challenging.
Although less than twenty cell and gene therapies had received regulatory approval by the end of 2019 in the EU, in early 2020 there were 1,000 clinical trials underway in over 400 companies (1;2), therefore Payers/HTA need to quickly find ways to evolve their decision-making processes to help resolve uncertainties and mitigate risks. One option is to consider a longitudinal approach to evidence generation with collection of real-world data (RWD) over the life cycle of the technology (Reference Annemans3).
The potential to use RWD to make decisions about highly innovative technologies links into the major scientific and technological advancements in digital health (4;5). Numerous national and transnational collaborations are expected to contribute to improvements in quality, coverage, and access to RWD (Reference Plueschke, McGettigan, Pacurariu, Kurz and Cave6–8) and have the potential to create learning health systems that will improve patient outcomes (9). The health technology industry, health systems, clinicians, patients, and methodologists are working to improve use of real-world/big data in the healthcare sector. However, views differ on how RWD can be used and the potential to generate robust reliable real-world evidence (RWE) to inform major decisions.
Regulators have used RWE for many years for pharmacovigilance and have recently developed frameworks for use of RWE in wider regulatory contexts, but continue to state that evidence from randomized controlled trials (RCTs) remains the best available standard for demonstration of efficacy and such data should be seen as complementary to RCT evidence (7;10). Medical and regulatory leaders across the EU (11) have highlighted that the evidence requirements for each regulatory decision are not the same. Acceptability is influenced by the question being asked, the level of risk and other considerations such as the ability to capture other data, availability of other treatments, and unmet medical need. Such contextual considerations also influence Payer/HTA views on the persuasiveness of RWE (12;13). In the U.S., it has been stated that demonstration of superiority, expansion of use, contradiction with RCT results or major change in clinical practice will all require a “high evidence bar.” Whereas assertion of equivalent effectiveness and lower cost, a new safety signal and complementing RCT data to fill gaps may all be “low evidence bar” situations (Reference Pearson, Dreitlein, Towse, Hampson and Henshall14). Both regulators and Payers/HTA agree that acceptability of RWE is dependent on data and methodological processes to control for bias and confounding.
In 2017, the CEO (de Cock) of the National Institute for Health and Disability Insurance-INAMI/RIZIV (the Belgian healthcare payer) initiated a series of multi-stakeholder initiatives to explore whether robust RWE could be obtained to close evidentiary gaps in Payer/HTA decisions, particularly in relation to rare disease treatments. The resulting reports included principles for use of RWD over the entire technology life cycle (Reference Annemans3) and for Outcomes-Based Managed Entry Agreements (OB-MEA) (Reference Annemans and Pani15). Annemans and Makady (Reference Annemans and Makady16) reported on the TRUST4RD initiative, producing a Tool for Reducing Uncertainties in the evidence generation for Specialized Treatments for Rare Diseases. It included a taxonomy of evidence/uncertainties that arise with rare disease treatments to support iterative multi-stakeholder dialogues and recognized that RWE may be able to resolve uncertainties about natural history, the interaction between the technology and the disease (including long-term treatment effects), and uncertainties related to the healthcare ecosystem.
Although these were multi-stakeholder initiatives with broad buy-in, there was still a lack of uptake and little advancement in use of RWD for decision making. Hence, it was decided to consider how these three initiatives and other ongoing RWD policy activities could be applied to the challenges associated with Payer/HTA decisions for highly innovative treatments, which was an issue that loomed large in 2019.
This initiative, RWE4Decisions was commissioned by INAMI/RIZIV to explore with all stakeholders the potential use of RWD in this specific case of making Payer/HTA decisions about highly innovative technologies and agree clear actions. It excluded consideration of underpinning issues about data sources, methodologies, governance, analytics, and infrastructure that were being addressed by others (6;17).
This paper explains the methods used and the resulting vision and key principles. It outlines key stakeholder roles in relation to RWD and proposes actions for each stakeholder group to test the use of RWD to inform Payer/HTA decisions about highly innovative technologies. Finally, consideration is given to how a multi-stakeholder learning network for RWE can be established to align work with other initiatives, share best practices, and develop guidance that will ultimately lead to optimization of treatment and outcomes through best use of RWD.
Methods
All work was undertaken according to the “Bad Gastein” principle of equal representation of all stakeholders from the fields of public health and health care (18). Representatives from HTA bodies, payers, regulators, government bodies, clinical research organizations, pharmaceutical industry, patient groups, and academia participated in meetings and email consultations (the Review Group) and a sub-group involving all stakeholders brainstormed ideas arising between each step via teleconference (the Task Force). This work linked into the EU Finnish Presidency program in 2019 on the “economy of well-being” that included discussion about using RWD for evaluation of the value of pharmaceuticals, and included consultation as outlined below.
A mixed-methods approach was used that explored recent policy proposals about use of RWD applied to the specific context of Payer/HTA decisions about highly innovative technologies and developed feasible actions that were implementable by each stakeholder group. Workshops, teleconference, and email consultations were used over several months as follows:
• Presentations from the Finnish Health/Pharmaceutical attaché to the EU, a payer collaborative, HTA bodies, and a European clinical network explored the challenges with use of RWE to resolve the Payer/HTA uncertainties emerging with highly innovative technologies. A roundtable discussion drawing in European patient groups and industry representatives discussed what practical steps could be taken.
• Desk top research—Selected policy documents from the EU and U.S. about the use of RWD in regulatory and HTA decision making published from January 2018 to April 2019 were reviewed and discussed to avoid “reinventing the wheel”.
• Case studies—Members of the initiative were asked to put forward case studies of marketed highly innovative technologies where RWE was pivotal to reimbursement decisions or where challenges in RWD collection remained. This resulted in one case study from HTA (relating to nusinersen), one from a Payer (relating to tisagenlecleucel) and two confidential topics from industry.
• Development of stakeholder actions—After the case study workshop, each individual wrote down actions they felt were needed by each stakeholder to make consistent use of RWD in Payer/HTA decisions become a reality. Actions were discussed on the day, then amalgamated, and refined by the Taskforce and their networks over several months, including a presentation to the EU Chief Pharmaceutical Officers.
• Version 1 of the stakeholder roles and actions were issued publicly for 3 months consultation with a specific request to encourage European stakeholder groups to respond. The actions were also presented at international meetings for feedback. Detailed feedback was received from eleven expert groups and major revisions were made to the actions. The revised actions were then reviewed by the Task Force and finalized by the authors.
Meetings were chaired by de Cock and an ex EU parliamentarian. Facey undertook the desktop research, facilitated the workshop, and helped prepare materials with Batchelor and Borchardt who organized all meetings. Rannanheimo was the key link to the Finnish EU Presidency and provided substantial input to the redrafting of the recommended actions and learning network proposals.
Results
The Potential for Use of RWD in Decision Making in the EU
The initial stakeholder presentations and discussions with stakeholders identified that EU Member States (MS) differ markedly in their digital health capabilities. Some have excellent electronic health (and other government) records that can be linked via a unique patient identifier (such as Finland, Scotland, Norway, Sweden, and France), but analyses can be cumbersome and key information such as diagnosis may not be recorded on prescriptions. Other MS have developed bespoke registries to monitor appropriate utilization of new pharmaceuticals and enable OB-MEA (such as Italy and Portugal). Hence, the landscape for RWD collection is disparate across the EU and the infrastructure and governance frameworks to enable data sharing across EU MS do not yet exist. Initiatives are underway to address these concerns (8;11) at an international policy level, but these are complex and will take time. Therefore, this work focused on the specific issue of making pricing and reimbursement decisions about highly innovative technologies, seeking to develop feasible actions for each stakeholder at a national or transnational level that could be enacted now and in the near future.
The desktop research identified recent initiatives to support use of RWE in healthcare decision making. These all highlighted the need for developments in data governance to enable appropriate data sharing according to data protection legislation and robust methodological approaches to address biases. Key elements about the potential use of RWD were interpreted for the Payer/HTA decision-making context as shown in Table 1.
Table 1. Stakeholder views on RWE in decision making
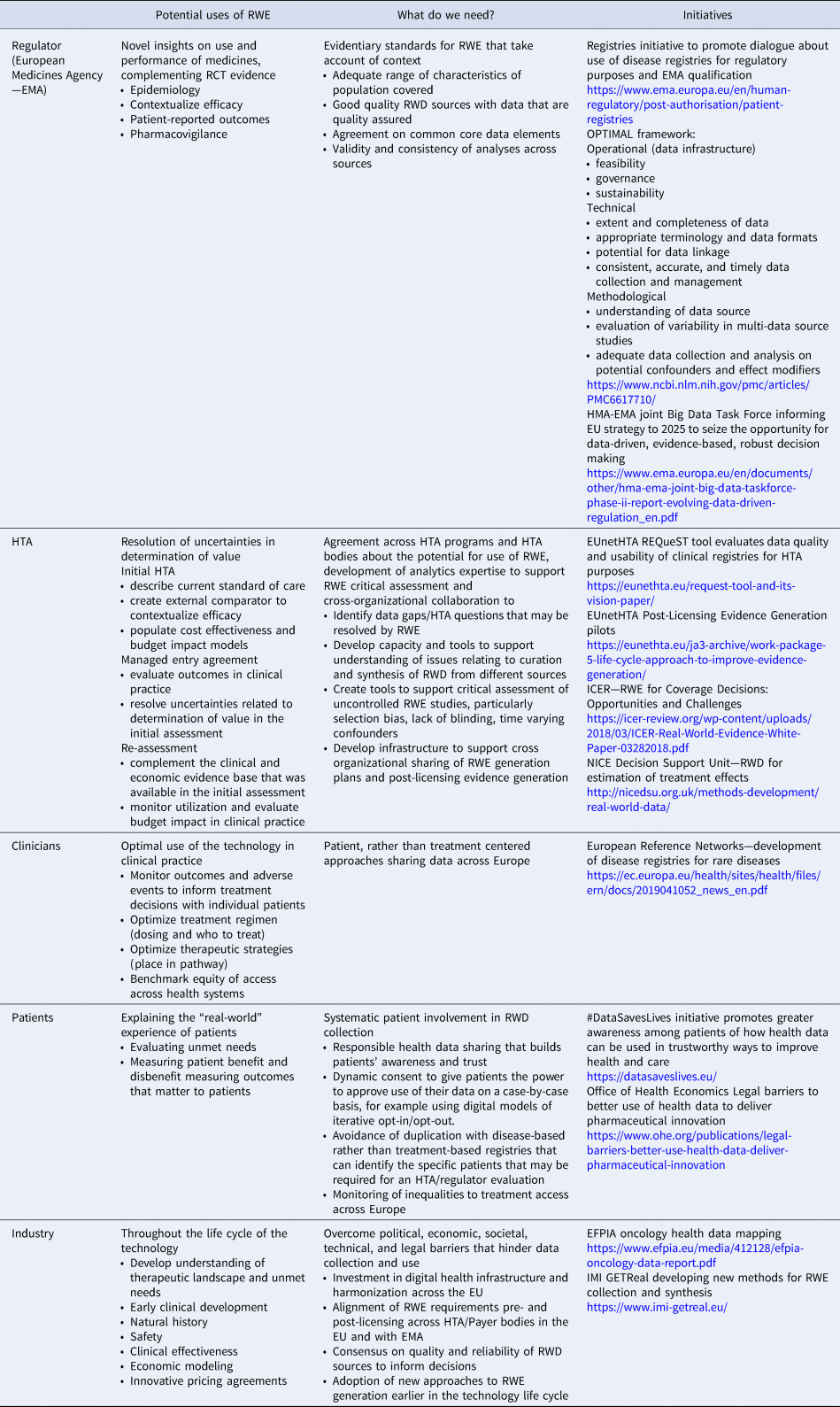
EFPIA, European Federation of Pharmaceutical Industries and Associations; EMA, European Medicines Agency; EU, European Union; HMA, Heads of Medicines Agencies; EUnetHTA, EU network for HTA; HTA, health technology assessment; ICER, Institute for Clinical and Economic Review; NICE, National Institute for Health and Care Excellence; RWD, real-world data; RWE, real-world evidence.
RWE4Decisions—Actions for Each Stakeholder Group
Case studies were discussed in multi-stakeholder workshops, to see if existing initiatives provided sufficient guidance to inform Payer/HTA discussions about RWD. All stakeholders agreed that the large number of initiatives showed fragmentation of approaches with divergent guidance emerging. Siloed working had led to a lack of clarity about what RWD might be suitable for what questions and how that could be developed into robust RWE to inform Payer decisions. This led to the recognition by all stakeholder groups that collaboration is essential and that this must be underpinned by trust and transparency as outlined in the vision and key principles (Figure 1). To ensure that development of RWE is seen as a shared responsibility the roles of each stakeholder to support the generation, analysis and interpretation of RWD to inform decision making were clarified as outlined in Table 2.
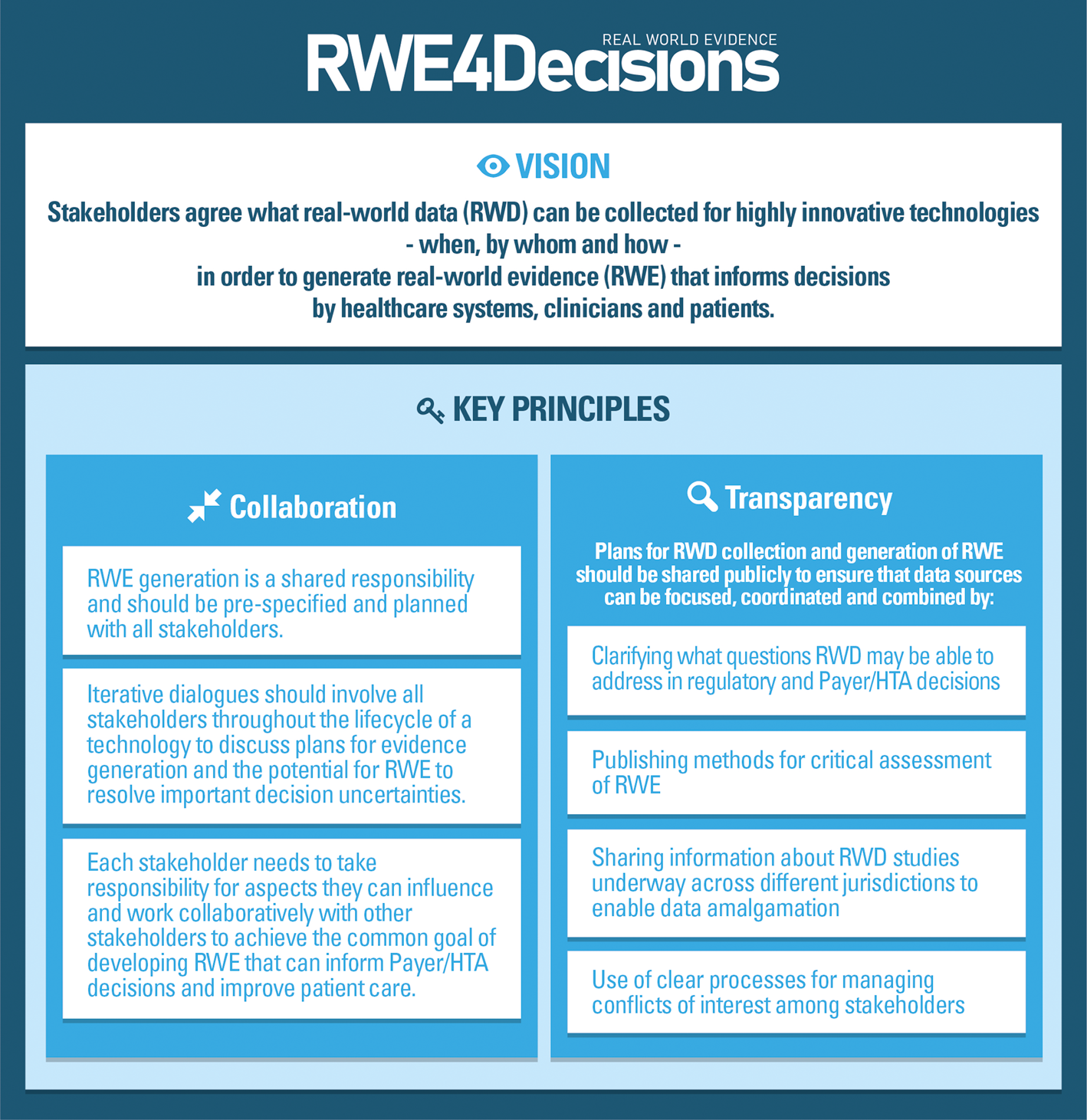
Figure 1. RWE4Decisions vision and principles.
Table 2. Stakeholder roles in relation to use of RWD in decision making

a EMA uses the term “registry coordinator”—a person or entity having a role in the overall coordination of a registry or of a platform of several registries. Registry holders can be patient groups, healthcare professionals, clinical institutes, and manufacturers.
b These groups could be within the health sector, academia, or private sector consultancies.
Working within the principles of collaboration and transparency, each stakeholder can undertake actions to support the use of RWE in Payer/HTA decisions about highly innovative technologies. Table 3 presents the recommended actions for each stakeholder group that have been modified over several iterations with the Taksforce, Review Group, and public consultation, with final amendments by the authors. These recommendations are more fulsome for Payer/HTA bodies and pick up on recommendations made in ongoing initiatives by European regulators, multi-stakeholder groups.
Table 3. RWE4Decisions stakeholder actions
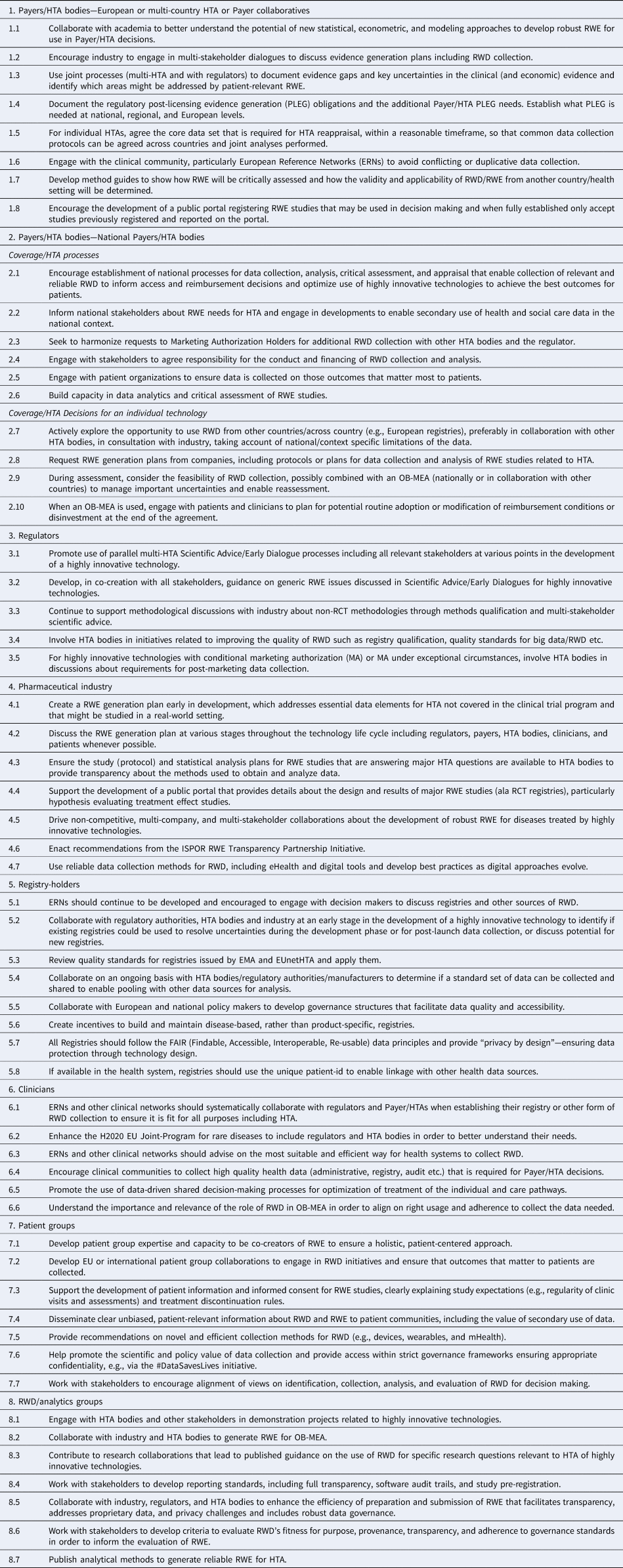
ERNs, European reference networks; FAIR, findable accessible interoperable re-usable; MA, marketing authorization; OB-MEA, outcomes-based managed entry agreement; PLEG, post-licensing evidence generation; RCT, randomized controlled trials; RWD, real-world data; RWE, real-world evidence.
Discussion
In this initiative, case studies of recent Payer/HTAs about highly innovative technologies were considered against recent policy proposals relating to RWD. This showed a lack of clarity about the Payer/HTA questions that could be answered by RWD and how the quality of RWE could be assessed. All stakeholders worked together to create a vision whereby stakeholders agree what RWD can be collected for highly innovative technologies based on principles of collaboration and transparency. For each stakeholder group, recommended actions to support the generation, analysis, and interpretation of RWD to inform decision making were developed.
At the outset of this work, the intention was to provide a one-page quick reference guide highlighting key actions that could be taken to develop RWE for highly innovative technologies. After the case studies, actions needed by HTA/Payers, regulatory bodies and industry were proposed. In this activity individuals identified that patient groups, registry holders, and clinicians should also take responsibility for certain actions and so they were developed. In consultation, it became apparent that the stakeholder group of RWD/analytics organizations was missing. Actions were developed for this group based on the comments of the consultee and consulted upon with another analytics organization and the Taskforce. Hence, rather than a one-pager, a list of actions was created for each stakeholder group. This is important because central to the RWE4Decisions vision is that development of robust RWE is a shared responsibility.
In Europe, collaboration across Members States is also essential, particular for very rare diseases, where study in one country will be insufficient.
The EU's new data strategy (19) promotes data-driven innovation to bring major benefits to citizens. Health is acknowledged as one of the sectors where Europe can benefit from the data revolution: improving the quality of health care, while decreasing costs, but this requires the right governance mechanisms to be put in place. To enable this, the European Commission (EC) will propose legislative or non-legislative measures for development of a European Health Data Space to promote health data exchange and support better diagnosis, care, and outcomes for patients. It is an essential step for informed, evidence-based decisions to improve the accessibility, effectiveness, and sustainability of the healthcare systems (20).
In the EU, public–private partnerships, like the IMI Big Data for Better Outcomes projects provide important test beds for collation of data in a particular medical field and proof of concept for how data can be effectively shared to answer clinical questions. The GetReal Initiative has focused on methods development for RWD to inform both regulatory and HTA decision making and is seeking a sustainable platform.
RWE4Decisions has focused on the EU given the imperative for cross border collaboration, but internationally other RWD initiatives are emerging that will be important to link with.
The U.S. FDA's Sentinel program, involving over 100 million individuals, is a trusted source for pharmacovigilance. Building on that experience the FDA has created a RWE program that will provide guidance on the relevance and reliability of RWD and the design and analysis of effectiveness studies to inform regulatory decisions. This will include demonstration projects and involve the FDA leadership to promote shared learnings and consistency of approaches across the Agency (7).
The World Health Organization has stated that digital health initiatives need to be underpinned by a robust strategy that integrates financial, organizational, human, and technological resources (8), recognizing legal constraints and national contexts. Ideally, a legal framework, platform, and governance processes are needed that provide access to health data across Europe via appropriate governance mechanisms for bona fide research and decision-making purposes, taking into consideration public concerns about commercialization of patient and health system data. We are some distance away from that vision, but areas of progress are seen. The eHealth Digital Service Infrastructure enables sharing of prescription data and patient summaries between MS (20). Although only a small number of countries are currently involved, contribution, and experience is building that will enhance future learning and collaboration.
Essential to the RWE4Decision vision is that there is transparency in RWE generation. This is not simple given the need to maintain a competitive advantage that is obvious in any industry. However, the ISPOR RWE transparency taskforce is tackling this issue and will produce important findings agreed by all stakeholders in the future.
In 2019, the HTAi Global Policy Forum noted that despite the promise of RWD, the HTA community is not yet fully equipped to address the challenges of using RWD (Reference Oortwijn, Sampietro-Colom and Trowman21). They called for more clarity on methods, standards, streamlined collection of RWD, data sharing across jurisdictions, replication of RWD, and expert analysis. It suggested that the HTA community should develop actions and guidance to develop and manage RWD/RWE to inform decision making. This paper seeks to respond to that by choosing a challenging area for Payers/HTA that could be informed by RWE and proposing actions for each stakeholder group. However, this is not enough. We need to embody continuous improvement cycles, reflecting on attempted actions, modifying them with experience. As a result, INAMI has undertaken a workshop to provide informal advice on highly innovative technologies that are in development, taking account of the stakeholder actions, seeking to develop general learnings that can be shared. Other stakeholders are socializing RWE4Decisions in their networks and seeking link implementation with other initiatives such as #DataSavesLives.
A Multi-Stakeholder EU Learning Network on RWE
The Heads of Medicines Agency (HMA) and EMA have indicated that to develop use of Big Data in health, iterative approaches are needed with relevant stakeholders. This should support data standardization and improved data quality, promotion of data sharing and access, then robust data processing and analyses may be possible to produce RWE that has regulatory acceptability (22). To develop this work, they have issued ten recommendations that include delivery of a sustainable platform to access and analyze healthcare data from across the EU (DARWIN) (11). This is essential to enable cross border data sharing. An EU Big Data “stakeholder implementation forum” is also proposed to build a resource of key messages and communication materials on regulation and Big Data. The RWE4Decisions initiative is keen to see the development of this forum and following the multi-stakeholder work of INAMI over recent years, it is hoped that it will follow the “Bad Gastein” principle in terms of breadth of participation to be a collaborative “Learning Network.” This could use the EC's Open Innovation 2.0 approach (23;24), where all stakeholders work together to enable cross-fertilization of ideas to develop innovation far beyond the scope of what one organization or initiative can do alone. Järvensivu (25) provides guidance on learning, working, and leading together in networks, which we have used to develop principles for a Learning Network as outlined in Box 1.
Box 1. Developing an effective learning network
Key principles
A Learning Network needs to:
• be premised on open governance, reciprocity, and legitimacy through comprehensive membership
• enable knowledge sharing and dialogue, and operate on the basis of shared responsibilities and clear roles
• be able to develop actions, to reach goals efficiently, and be able to question goals and practices to develop new learning methods
• enable the attainment of common goals, develop network members' own work, skill, and capabilities
In order to deliver on the goals, the Learning Network must be:
• Owned by a public institution
• Enabling the multi-stakeholder interaction
• Sustainable through long-term funding.
The RWE4Decisions initiative can do its part in a multi-stakeholder EU Learning Network for RWE by continuing to bring together policy makers, payers, HTA bodies, regulatory bodies, clinicians, patient groups, industry, and academic experts to share expertise as they try to implement these stakeholder actions. Keeping abreast of initiatives in other groups, piloting new processes with decision makers, discussing implementation challenges, and reflecting on challenges and successes can all contribute to outputs and continuous learning to realize improvements in the use of RWE to inform decisions about highly innovative technologies. This is particularly important for areas where there is high unmet need and where EU cross border collaboration is most needed, in the area of rare diseases.
Conclusions
Highly innovative technologies make a good case study to consider how RWE can be developed through iterative multi-stakeholder dialogues to inform decisions by Payers/HTA bodies. All stakeholders need to be involved, to agree what RWD is needed for what purpose, how it can be collected, when, by whom and how. This includes considerations of cross-border (cross-health system) HTA/regulatory collaboration to agree RWD requirements and the associated infrastructure, development of data analytics methods for HTA, and transparency in RWE studies akin to that of RCTs. Demonstration projects on highly innovative technologies could realize some of the stakeholder actions presented in this paper, start to deliver some of the outputs, and inform how new processes can be developed. This will provide substantial insights to a wider learning network that could develop systems to support a learning health system, optimization of health technology use, and improvement in patient outcomes through best use of RWD.
Acknowledgments
The following individuals were members of the Review Group that contributed to the exploration of the issues and development of the stakeholder actions presented in this paper: Lieven Annemans, University of Ghent; Simone Boselli, EURORDIS; Simon Butler, Gilead Sciences; Antonella Cardone, European Cancer Patient Coalition; Marc van de Casteele, INAMI; Ivana Cattaneo, Novartis; Mercedes Echauri, Takeda; Marie-Hélène Fandel, Amgen; Ansgar Hebborn, Hoffman La Roche; Frank Hulstaert, Belgian Health Care Knowledge Centre; Kaisa Immonen, European Patients' Forum; Xavier Kurz, EMA; Denis Lacombe, European Organisation for Research and Treatment of Cancer; Amr Makady, formerly Dutch National Healthcare Institute (ZIN); Alexander Natz, European Confederation of Pharmaceutical Entrepreneurs (EUCOPE); Ulla Närhi, Finnish Permanent Representation to the EU; Patrick Neven, UZ Leuven; Aisling O'Leary, National Centre for Pharmacoeconomics (Ireland); Delphine Roulland, formerly EUCOPE; Ad Schuurman, European Medicines Agency; Jennifer Shum, AstraZeneca; Timon Sibma, ZIN; Chris Sotirelis, EMA Patient Expert; Bjørn Oddvar Strøm, Norwegian Medicines Agency; Lonneke Timmers, ZIN; Sheela Upadhyaya, NICE; Entela Xoxi, Catholic University of Rome.
Source of Funding
This work was financially supported by EUCOPE, Amgen, AstraZeneca, Gilead Sciences, Hoffman-La Roche, Takeda, and Novartis which covered the costs of the multi-stakeholder meetings, the work of FIPRA, and KF for workshop facilitation. Some workshop participants received travel expenses. KF undertook other elements, including all consultation and writing activities as part of her work in the EC H2020 IMPACT HTA project (grant number RE8213). PR and JdeC undertook all work independently under the auspices of their employers.
Conflict of interest
Dr. Facey reports personal fees from FIPRA and grants from EC H2020, during the conduct of the study. Ms. Rannanheimo reports no personal grants; Fimea is the national competent authority for regulating pharmaceuticals in Finland and also a partner organization in EUnetHTA. Ms. Batchelor and Ms. Borchardt report grants from Amgen, Gilead Sciences, Astra Zeneca, Novartis, Takeda, Hoffmann-La Roche, and EUCOPE (European Confederation of Pharmaceutical Entrepreneurs), during the conduct of the study. Mr. De Cock has nothing to disclose.






