Diabetes care programs increasingly include advanced mobile-based technological devices. These technologies entered the market aiming to supplement traditional diabetes healthcare and to support self-management by patients (Reference Hunt1). Consequently, smart applications on mobile devices for diabetes self-management proliferated, increasing exponentially in app stores (2). Diabetes apps are designed to support self-management activities like blood glucose (BG) and complication monitoring, medication adherence, healthy eating, exercise, and problem-solving (Reference Hunt1). With the increased availability of apps targeted at consumers, research began examining their potential for diabetes self-management.
Current trends in mHealth, “the use of mobile communications for health information and services” (Reference Nacinovich3, p. 1), focus on effects research (Reference Free, Phillips and Galli4), contrasting overly optimistic study results on diabetes app use effects (Reference Wu, Yao and Vespasiani5) with a more critical view toward mHealth effectiveness (Reference Fu, McMahon, Gross, Adam and Wyman6;Reference Veazie, Winchell and Gilbert7). A meta-analysis showed that such interventions “that have statistically significant effects are small and of borderline clinical importance” (Reference Free, Phillips and Galli4, p. 25). The potential of mHealth for diabetes self-management, including diabetes apps, requires further investigation, especially in theoretical terms. We focus on the concept of empowerment as a fundamental predictor of self-management behaviors (Reference Yang, Hsue and Lou8), particularly in relation to diabetes (Reference Asimakopoulou, Gilbert, Newton and Scambler9;Reference Funnell and Anderson10), to evaluate the potential of diabetes apps for empowered self-management. Specifically, this study examines how technological features of apps for diabetes self-management correspond with theoretical indicators of (RQ1) psychological and (RQ2) behavioral empowerment.
The paper first defines two sub-concepts of empowerment for diabetes self-management, then addresses extant gaps regarding empowerment in mHealth research, particularly in relation to diabetes apps. To address research gaps, we examined features of 121 diabetes apps corresponding to indicators of empowerment.
Diabetes Self-Management and Empowerment
The introduction of home BG monitoring possibilities in the 1970s led to a shift in responsibility from healthcare professionals (HCPs) to patients, emphasizing the relevance of self-management in diabetes care (Reference Snoek11). Clark and Houle define patient self-management as “the conscious use of strategies to manipulate situations to reduce the impact of disease on daily life” (Reference Clark, Houle, Shumaker, Ockene and Riekert12, p. 27). Gomersall et al. (Reference Gomersall, Madill and Summers13), in a meta-synthesis of thirty-eight papers, report that type 1 and type 2 diabetes self-management (T1DM/T2DM) includes BG testing, medication adherence, regular exercise and the adoption of specific diets (compare AADE7 Self-Care Behaviors) (Reference Beck, Greenwood and Blanton14). The recent 2017 National Standards state that it is necessary to learn how to self-manage diabetes to prevent or to delay complications (Reference Beck, Greenwood and Blanton14). If self-management is poor over time, the risk for potential complications increases (15).
A growing body of literature notes the relevance of empowerment for diabetes self-management (Reference Tol, Alhani, Shojaeazadeh, Sharifirad and Moazam16;Reference Meer17). According to Gutschoven and van den Bulck (Reference Gutschoven and van den Bulck18), “(…) empowerment is expected to enhance the capacity for self-management and to promote the adoption of healthier lifestyle” (Reference Gutschoven and van den Bulck18, p. 7). However, a variety of interpretations leave an unclear understanding of empowerment (Reference Asimakopoulou, Gilbert, Newton and Scambler9). Different scientific disciplines offer varied conceptualizations, with an overarching agreement regarding empowerment as a motivational construct (Reference Schulz and Nakamoto19;Reference Spreitzer20), including management research (Reference Thomas and Velthouse21), community psychology (Reference Rappaport22), and clinical practice (e.g., Reference Funnell, Anderson and Arnold23).
Rappaport describes a multilevel construct comprising a combination of “psychological empowerment” for the individual, and social influence from others (Reference Lee and Koh24), referred to as “behavioral empowerment” (or “role empowerment”) (Reference Logan and Ganster25). While somewhat similar to the concepts of intrinsic and extrinsic motivation, empowerment is a unique concept (Reference Lee and Koh24).
First, early approaches in management studies (Reference Conger and Kanungo26) and in diabetes research (Reference Anderson, Funnell, Fitzgerald and Marrero27) comprehended psychological empowerment as related to self-efficacy, the “belief in one's agentive capabilities, that one can produce given levels of attainment” (Reference Bandura28, p. 382). Thomas and Velthouse (Reference Thomas and Velthouse21) noted that such a one-dimensional approach did not go far enough, and argued for a multidimensional approach to psychological empowerment, based on four empowerment indicators of perceived meaningfulness (relevance), perceived competence (self-efficacy), self-determination (choice), and perceived impact.
Schulz and Nakamoto (Reference Schulz and Nakamoto19) translated these indicators to a health context. According to them, perceived relevance suggests that the health activities the patient performs are seen as worthy, potentially leading to higher commitment, while perceived competence describes confidence about the ability to manage one's own health condition. Self-determination concerns the possibility of actions initiated by the patients themselves, and, perceived impact comprises feelings about making a difference in health outcomes, for example, exercises that result in weight loss.
Second, behavioral empowerment, or the empowering support by others, has been shown to influence the feeling of psychological empowerment (Reference Logan and Ganster25;Reference Oh and Lee29). In a diabetes care context, social support can derive from HCPs, being the first source of professional medical support for patients (e.g., diabetes educators, Reference Burke, Sherr and Lipman30), or from private social patient networks (Reference Rosland, Kieffer and Israel31;Reference Heisler, Vijan, Makki and Piette32).
For the former, Emanuel and Emanuel (Reference Emanuel and Emanuel33) suggest different models of the doctor-patient relationship with varying HCP decision-making styles defined as “the propensity of physicians to involve patients in treatment decisions” (Reference Heisler, Bouknight, Hayward, Smith and Kerr34, p. 246). The ideal empowering doctor-patient relationship comprises neither exclusive control by the physician (”paternalistic” model) nor absolute autonomy by the patient (”informative” model), but a collaborative process of shared decision making with active contribution by both parties (“deliberative” model, Reference Emanuel and Emanuel33). Shared HCP decision-making, empowering patients (Reference Schulz and Nakamoto19), can be considered an indicator of behavioral empowerment. Likewise, communication is part of HCP–patient interaction (Reference Roter and Hall35), and HCPs can empower patients by willingly sharing greater medical information (Reference Omboni, Caserini and Coronetti36).
The latter case of support by members of the private social patient networks on self-management outcomes (Reference Whitehead, Jacob, Towell, Abu-Qamar and Cole-Heath37;Reference Strom and Egede38) has been compared with professional HCP support (Reference Rosland, Kieffer and Israel31;Reference Heisler, Vijan, Makki and Piette32). Isaksson et al. (Reference Isaksson, Hajdarevic, Abramsson, Stenvall and Hornsten39) found that higher psychological empowerment was not just associated with support from HCPs but also support from relatives. Shao et al. (Reference Shao, Liang, Shi, Wan and Yu40) found that support by private social networks was positively associated with self-efficacy in diabetics, and that self-efficacy mediated the relationship between support and glycemic control.
To summarize, two dimensions of empowerment, psychological (Reference Tol, Alhani, Shojaeazadeh, Sharifirad and Moazam16;Reference Kleier and Dittman41;Reference Camerini, Schulz and Nakamoto42) and behavioral (Reference Strom and Egede38;Reference Grant and Schmittdiel43;Reference Bennich, Roder and Overgaard44), have ample empirical support as predictors of (diabetes) self-management behaviors as well as of health outcomes. Indicators of psychological empowerment include perceived relevance, perceived competence, self-determination, and perceived impact, while HCP decision-making, HCP–patient communication, and the social support by private patient networks are indicators of behavioral empowerment. We next address the missing consideration of empowerment in mHealth research (Reference Anshari, Almunawar and Adibi45).
Research Gaps linking Empowerment and App Features
Despite literature discussing the potential of apps for diabetes self-management (Reference El-Gayar, Timsina, Nawar and Eid46), specific conclusions for empowerment, lacking empirical evidence, rarely go beyond general overviews (Reference Anshari, Almunawar and Adibi45;Reference Krošel, Švegl, Vidmar, Dinevski and Bonney47), or are not based on a comprehensive theoretical explication (e.g., Reference Park, Burford, Lee and Toy48). Most diabetes-related mHealth projects focusing on empowerment are of applied character and/or do not have much explanatory value (Reference Park, Burford, Lee and Toy48–Reference Cumming, Strnadová, Knox and Parmenter50). Studies of empowerment in mHealth practice are both limited and inconclusive (e.g., Reference Bradway, Arsand and Grottland49;Reference Cumming, Strnadová, Knox and Parmenter50). Conceptually, empowerment is mainly understood as an outcome of mHealth use (Reference Park, Burford, Lee and Toy48;Reference Bradway, Arsand and Grottland49). For example, Park et al. (Reference Park, Burford and Hanlen51) found that empowerment could occur when T2DM patients shared information or received social support using their mobile devices, when patients realized the outcomes of mHealth supported activities, or when the mobile devices were used for improved activity planning. Krošel et al. suggest that mHealth is “(…) offering different means for introduction of the concept of empowerment into patients’ everyday life” (Reference Krošel, Švegl, Vidmar, Dinevski and Bonney47, p. 35), yet conclude it still plays a minor role in diabetes self-management, with apps lacking perceived benefit and ease of use.
There is a limited literature relating mHealth use to psychological empowerment outcomes (Reference Li, Owen, Thimbleby, Sun, Rau, Beuscart-Zéphir, Jaspers, Kuziemsky, Nøhr and Aarts52;Reference Mantwill, Fiordelli, Ludolph and Schulz53), with the associated Web-based eHealth literature proving inconclusive, both finding effects of food label-training (Reference Miller, Sutter and Wilson54) and failing to find any significant impact of functional eHealth interactivity (Reference Camerini and Schulz55) on psychological empowerment. On the other hand, the literature focusing on social support outcomes by using m/eHealth tools mostly does not refer to empowerment. Moreover, most studies did not examine social support as an outcome of mHealth use, but viewed social support delivered through mHealth influencing health and self-management outcomes (Reference Burner, Lam and DeRoss56).
We note that, while empowerment is conceptually under-studied in an mHealth context, a fair amount of research has focused on features of diabetes apps. Our study aimed to link these two streams of research.
Diabetes app features reported in previous research included insulin, diet, weight, exercise and medication recording, data export, data sharing and communication, data storage and analysis, reminders or automated feedback, education, and medication use (Reference Veazie, Winchell and Gilbert7;Reference Conway, Campbell, Forbes, Cunningham and Wake57–59). Demidowich et al. (Reference Demidowich, Lu, Tamler and Bloomgarden60) examined forty-two Android diabetes apps to find that 86 percent included BG recording, followed by medication tracking (45 percent), and insulin dose calculators (26 percent). Some diabetes apps could be connected to external devices like sensors or BG meters (Reference Heintzman61), or used cloud-based systems for health data storage and exchange (Reference Beckman, Reehorst and Henriksen62). Overall, most diabetes apps offered similar functionalities and combined at most two functions (Reference Arnhold, Quade and Kirch63), prompting Brzan et al. (Reference Brzan, Rotman, Pajnkihar and Klanjsek64) to comment that apps with a greater variety and combination of features were needed to attract long-term users.
El-Gayar et al. (Reference El-Gayar, Timsina, Nawar and Eid46) reported that “limitations of the applications include lack of personalized feedback; usability issues (…); and integration with patients and electronic health records” (Reference El-Gayar, Timsina, Nawar and Eid46, p. 247). The (composite) usability scores of apps were relatively low, showing an average score of 11.3 of thirty (Reference Demidowich, Lu, Tamler and Bloomgarden60). Fu et al. (Reference Fu, McMahon, Gross, Adam and Wyman6) pointed toward low satisfaction ratings in diabetes apps, while others found that apps did not cater to the needs of low literacy diabetics (Reference Rodriguez and Singh65). Rossi and Bigi (Reference Rossi and Bigi66) reported that diabetes apps “do not seem to be based on solid theoretical models (…), [nor] intended as devices to be integrated in the ecology of the doctor–patient relationship” (Reference Rossi and Bigi66, p. 1).
To address existing research gaps, we combined empowerment research with research on diabetes app features and investigated how diabetes app features corresponded with theoretical indicators of psychological and behavioral empowerment.
Methodology
Operationalization
We first collected available diabetes app types and features, adapting app coding schemes by Arnhold et al. (Reference Arnhold, Quade and Kirch63) and the Mobile App Rating Scale (MARS) app classification, which is based on 372 criteria for assessing apps from twenty-five published papers, conference proceedings, and online resources (Reference Hides, Kavanagh and Stoyanov67). The chosen app analysis method offers a high level of validity (Reference Arnhold, Quade and Kirch63), with the MARS seen as one of the most comprehensive tools for app analysis, having exhibited good internal consistency with α = .90, and inter-rater reliability of intraclass correlation = .79 (Reference Hides, Kavanagh and Stoyanov67;Reference Chavez, Fedele and Guo68). Next, using an interpretive and exploratory approach, app features were preliminarily assigned to theoretical psychological and behavioral indicators of empowerment, then compared versus prior research results. For example, features were expected to support perceived relevance of self-management behaviors when enhancing a diabetic's feeling that a specific behavior (e.g., app use) is worth investing energy in.
The final codebook contained the (i) information on the app search, (ii) background app information from respective app stores, (iii) app feature assessment, and (iv) app user target group.
App Collection Procedure
An app search, using the keywords diabetes, blood sugar, and glucose, was conducted by means of Apple App Store and Google Play, comprising 97 percent global market share (69), on two mobile Android and iOS devices from October 29 to November 7, 2015 by a trained researcher. Inclusion criteria were English free-to-download diabetes apps that were frequently downloaded and used by Singaporean end-users in 2015 (70). English is the most spoken language in Singaporean homes (71). Most app users use free-to-download apps (70), with paid apps accounting for .05 percent of app downloads (Reference Viennot, Garcia and Nieh72). App store ranking was used as an indicator for usage frequency due to lack of established criteria.
To mitigate reported country differences in commercial app stores (Reference Grundy, Wang and Bero73), an iPad (offering larger variety in search settings than an iPhone; iPad mini model ME276GP/A, iOS 9.1) with the latest operating system was registered anew with a dummy Singaporean account to ensure the inclusion of local search results (not necessary for Android; Samsung Galaxy S4, Android 4.3).
Similar to the app review by Arnhold et al. (Reference Arnhold, Quade and Kirch63) (wide variation in sample sizes in previous studies) (Reference Grundy, Wang and Bero73), the search included diabetes specific apps addressing both T1DM and T2DM, excluding apps not specifically designed for diabetics (such as calorie counters). We selected the first fifty apps listed for each search term, assuming them to be most frequently downloaded by users as prior research reported that the top 10 percent of most downloaded apps accounted for over 96 percent of the total downloads (Reference Viennot, Garcia and Nieh72) and that users searching for apps tended to pick the apps shown in the top positions (Reference Dogruel, Joeckel and Bowman74). Our study did not aim to provide full coverage of all available apps in 2015/2016 due to a dynamic app market, but rather be amenable to testing of the conceptual research questions. We acknowledge that app data get outdated quickly; however, a follow-up check in 2018 suggested that the diabetes self-management app market did not alter drastically since data collection in 2016.
After removal of duplicate apps and those that failed to meet inclusion criteria, the final app list contained 121 diabetes apps, with twenty-four iPad-specific apps, fifty-one iPhone apps, and forty-six Android apps for diabetes self-management (Table 1) that were coded using the developed codebook (available upon request). An additional twenty-two diabetes apps included diabetic recipes, magazines, and journals, which were recognized but not used for further coding due to a general lack of features (Table 1). The resulting app sample contained both international and Singaporean apps, with the majority of the apps offered by international developers.
Table 1. Diabetes App Collection Procedure Results
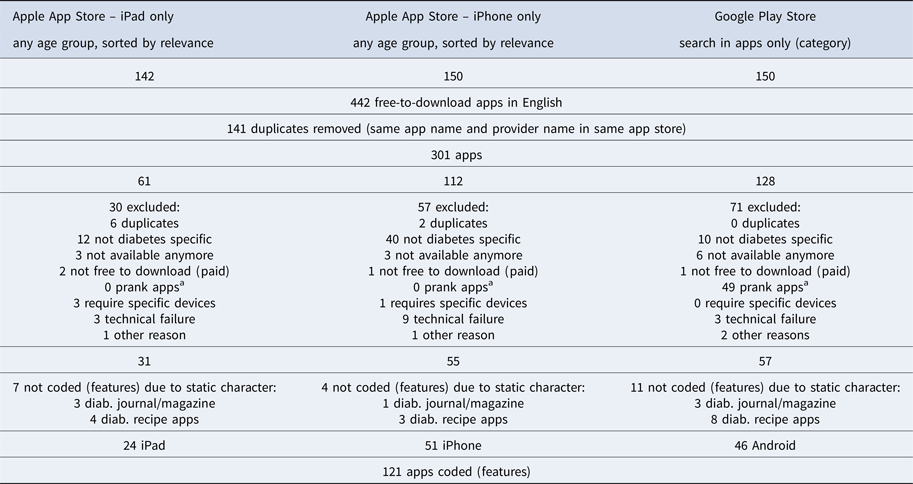
Note. a Prank apps are apps that just pretend to deliver a certain service (e.g., fake blood pressure measurement through the device screen).
Pretesting and Coding Procedure
Coders received training on the codebook and the procedure before the pretests and main coding. Several pretests on forty-five apps were run with three academic coders to ensure consistency. Inter-coder reliability (Reference Holsti75) had an acceptable average agreement of M = .82 (SD = .190; Min = .33; Max = 1.00) among all forty-three included variables. Sixteen variables showed full agreement of M = 1.00, fifteen variables an agreement of M = .83, eight variables an agreement of M = .67, one variable an agreement of M = .50, and three variables an agreement of M = .33.
The main app coding took place in February 2016 by three trained academic coders, using an Android smartphone, an iPad, and iPhones with the latest operating systems for coding (Coder 1: Mi4, Android 5.0; Coder 2: Apple iPad Air Wi-Fi-16GB, iOS 9.2.6 and iPhone6, iOS 9.2.1, Coder 3: iPhone 6, iOS 8.2).
Data Analysis
Based on previous app assessment (Reference Arnhold, Quade and Kirch63;Reference Hides, Kavanagh and Stoyanov67), feature availability was analyzed using numeric (mainly binary) and text variables. Coder comments were entered where additional information on the coded data was available. Ninety-nine was defined as missing data. Descriptive quantitative data analysis was undertaken in IBM SPSS version 25. An interpretive and qualitative exploratory analysis approach assigned app features to theoretical indicators of empowerment, confirmed by means of previous literature.
Results
Technological Features of Diabetes Apps
Almost two-thirds (62 percent) of the 121 apps analyzed included diabetes or health data logbooks for patients to record and analyze BG and other health data as part of diabetes monitoring (trackers/diaries, Figure 1). An eighth (12 percent) included learning tools and information apps for education. The sample contained BG or other data conversion calculators (7 percent, e.g., calculators to convert mmol/L to mg/dl) and diabetes community apps (3 percent, e.g., forum/chat apps). Marginal diabetes app categories comprised nutrition apps (e.g., databases for carbohydrate content in food) and exercise apps, and specific diabetes apps for children (logbooks) or gaming/quiz apps (Table 2; Figure 1). Table 2 provides an overview on types, target groups, and features of included diabetes apps (detailed data are available upon request).
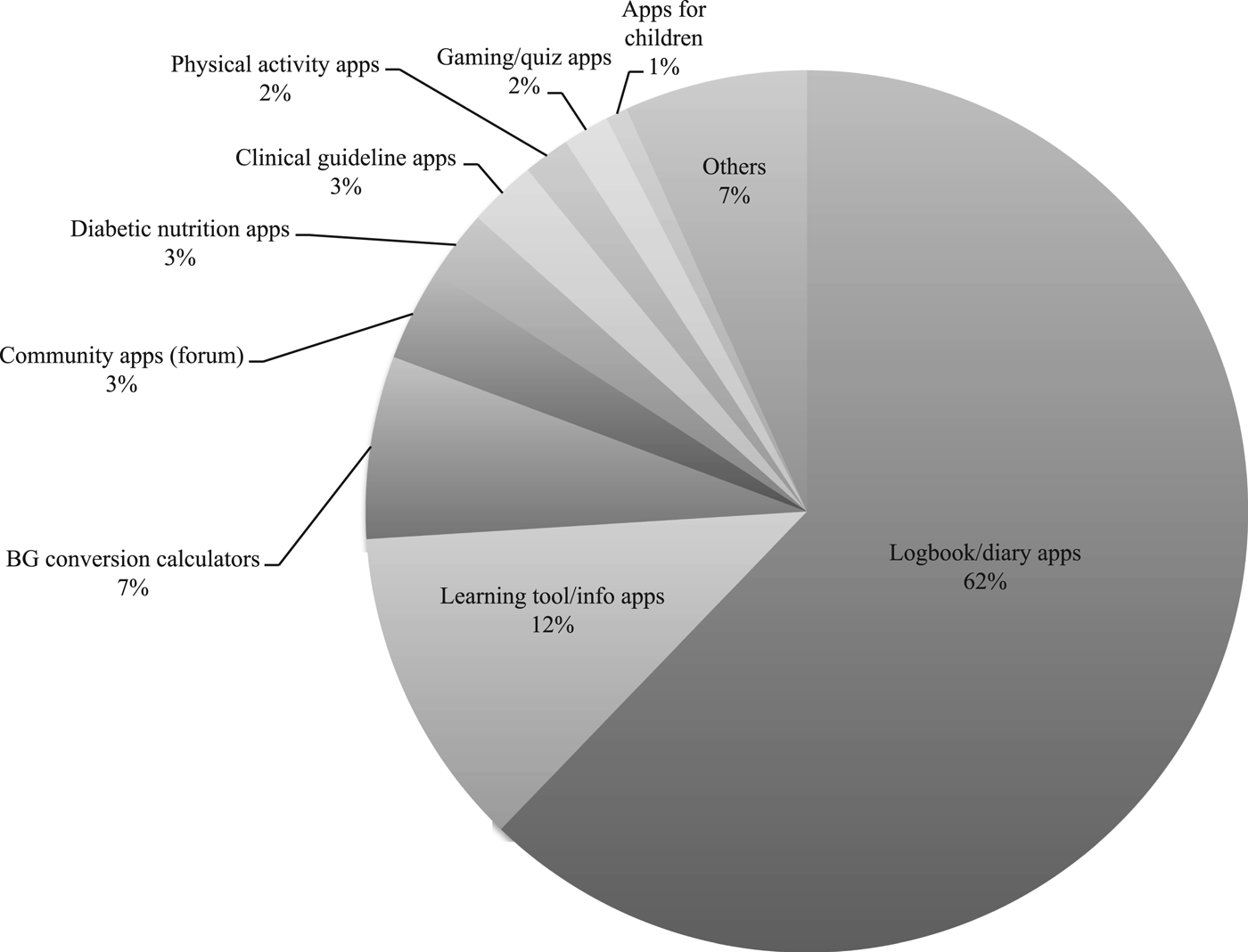
Fig. 1. Types of diabetes apps in the sample (N = 121).
Table 2. Diabetes App Types, Target Groups, Features, and Tailoring
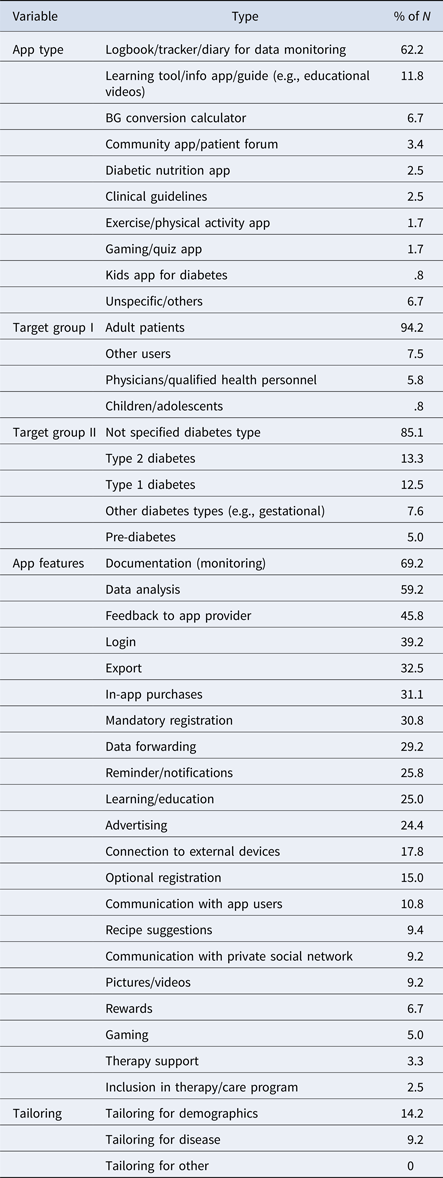
Note. N = 121, the values do not sum up to 100% because of partly mixed forms of apps.
App Features Corresponding to Psychological Empowerment Indicators
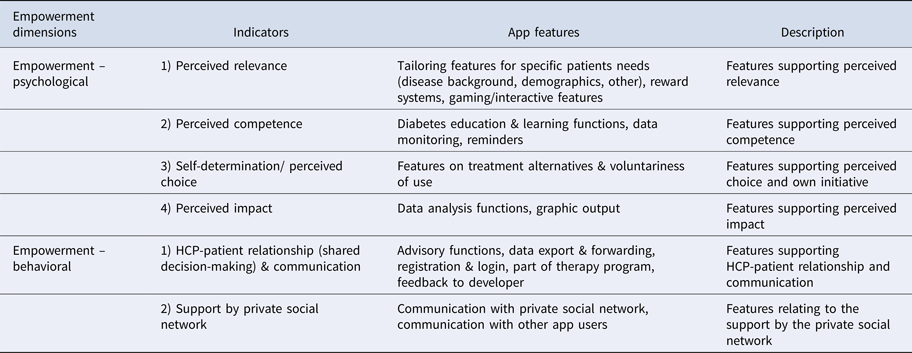
Specific app features (Table 2) corresponded specifically with theoretical indicators of empowerment (see Table 3 for link). App features such as customization, rewards, and interactivity were assigned to psychological empowerment indicators of perceived relevance; educational and data monitoring features (including reminders) were assigned to perceived competence, while analytical and graphic features were assigned to perceived impact.
Table 3. App Features Corresponding to Theoretical Empowerment Dimensions
Tailoring features appeared important for perceived relevance by providing an opportunity to individualize and adapt an app. Most apps analyzed failed to tailor services to specific patient subgroups with differing needs (e.g., young versus elderly). Eighty-five percent did not target any specific type of diabetic patient, but were meant for general use by all diabetics (Table 2). Only 13 percent of the analyzed apps specifically targeted T2DM patients, and an equal 13 percent targeted T1DM patients. A twelfth of apps had specific functionality for other forms of diabetes (8 percent) and a twentieth for prediabetics (5 percent). Adult diabetes patients were primary customers (4 percent), with a mere one percent aimed at diabetic children (Table 2). Furthermore, there was hardly any tailoring for disease characteristics, such the period since diagnosis, nor for demographic categories of age (in 14 percent of the apps, Table 2) and gender.
Reward features (7 percent) aligned with perceived relevance of diabetes apps, for example, bonus point systems for regular app use as part of a diabetes self-management regimen. These included interactive features like gaming elements (5 percent), possibly enhancing the relevance for specific target groups, such as adolescents.
Perceived competence for self-management was supported by app learning features that potentially enhanced diabetes knowledge. Improved knowledge could strengthen perceived diabetes self-management competence. A quarter of the apps (25 percent) provided features supporting learning processes and diabetes-specific knowledge through textual or video educational content (Table 2). We conclude that diabetes education was not the primary aim of most diabetes apps in the sample.
Facilitated data input through structured self-monitoring was assigned to the empowerment indicator perceived competence. Features for structuring BG documentation could promote improved diabetes monitoring, in turn leading to perceived competence for self-management. The available app features confirmed typical characteristics of diabetes logbooks, with a majority of apps (69 percent) supporting structured documentation of health data, such as storage of regularly measured BG values (Table 2). Documentation options included automatic data upload from the BG meter to the app by means of Bluetooth, taking pictures of the meter screen, or using the device keyboard to type BG and health data. Almost a fifth (18 percent) allowed a connection to external devices (e.g., BG meter, Table 2). Another quarter of the apps included reminders (to test BG) or automatic notifications (26 percent), that could support structured self-monitoring.
Features corresponding to perceived choice in self-management, such as information on various treatment alternatives, insulin options, or oral medication differences, were not found. There were few choices for the patient to feel better informed and better able to take own decisions. Regarding lack of perceived choice, no information was found on voluntary or obligatory use of apps as part of specific programs (e.g., DAFNE, Dose Adjustment For Normal Eating, http://www.dafneonline.co.uk). An obligatory use of diabetes apps could potentially hinder perceived choice regarding self-determined technology-supported self-management. However, an obligatory app use could also act as extrinsic motivation to regularize app use supporting diabetes self-management and, thus, create the necessity to use the app as part of an overall diabetes program.
Data monitoring was frequently accompanied by an option to analyze the entered data and/or to receive graphic data outputs (59 percent). Graphic data outputs and analysis features potentially support perceived impact of self-management activities. Such analytical features allow patients to interpret health data easily, and view outcomes of lifestyle changes. A visible improvement in BG values from app data could directly communicate effectiveness of self-monitoring to the patient. Thus, graphic and output features were expected to provide an opportunity to enhance the perceived impact of the app use.
App Features Corresponding to Behavioral Empowerment Indicators
Features that included apps in on-going diabetes programs (automatic data access for HCPs), forwarding and export features to provide HCPs with health and lifestyle data, and HCP contact information were assigned to the behavioral empowerment indicators shared decision-making and HCP–patient communication, and features to communicate with other users and the individual patient networks were assigned to the indicator of social support by private networks.
The results indicated that only a small percentage of apps (3 percent) were part of a larger therapeutic program for diabetes care (Table 2). While almost two-thirds of apps provided logbooks for data monitoring (62 percent, Figure 1), one-third were found to provide export features (33 percent) or data-forwarding features (29 percent). These features allowed the app user to provide HCPs with patient data, for example, by means of email or print (Table 2). Most apps did not enable automatic access to patient data for the HCPs, and thus user-friendliness of the data download and export options was likely to influence the perceived usefulness of such features.
Functionality for registration and log-in options made continuous data tracking and automatic data access complicated. Registration (mandatory 31 percent, optional 15 percent) and login-options (39 percent, Table 2) were partially available for communication and data storage. Few apps (3 percent) included direct contact to HCPs for therapy support or for advisory purposes (Table 2), for example, direct online feedback from HCPs by means of chat (e.g., with dieticians as found in Glyco app by Holmusk). Overall, diabetes apps lacked direct contact options with HCPs, failing to empower the patient through feedback, information, or motivational support.
Regarding social support as an aspect of behavioral empowerment, it was argued theoretically that features allowing information exchange with other diabetics, or with other patient networks, would promote online communication that could empower the user. However, only a ninth of diabetes apps included features to communicate either with other app users (11 percent), or within their own private user networks (9 percent, Table 2). Thus, the idea of social support by private patient networks was not promoted comprehensively in the app sample.
In summary, a limited range of app features were found that supported theoretical dimensions of psychological and behavioral empowerment. Innovative features were lacking, and features frequently appeared in a sub-section of the apps.
Discussion
The analytical review of app features corresponding to theoretical indicators revealed that the potential of the analyzed diabetes apps for empowerment is far from being realized. Empowerment considerations were limited to a small set of infrequently applied features (e.g., limited tailoring).
Previous research on app features assigned to indicators of psychological empowerment has shown that tailoring (as a means for perceived relevance) could enhance individual message relevance (Reference Kreuter and Wray76). Indeed, diabetes research has frequently demonstrated differences in self-management requirements in T1DM or T2DM patients (77). Likewise, differences in age groups requires diverse tailoring strategies for age specific content. Researchers and developers need to tailor app information more specifically to the type of diabetes and other characteristics of specific patient groups (Reference Thabrew, Stasiak, Garcia-Hoyos and Merry78). In particular, the lack of diabetic children as an app target group in the sample (Table 2) suggests that current app developers fail to realize the potential of technology for young “digital native” target groups highlighted by previous studies (Reference Lau, Lau, Wong and Ransdell79). A case for stronger inclusion of interactive elements for selected target groups can be made on the basis of prior mHealth studies on effects of gamification (Reference Lister, West, Cannon, Sax and Brodegard80;Reference Johnson, Deterding and Kuhn81), such as reward systems implemented in mobile games (Reference Lewis, Swartz and Lyons82).
The perceived competence indicator of psychological empowerment suggests that app features comprising educational elements and structured self-monitoring are important. Previous studies have found significant relationships between knowledge and perceived competence (e.g., Reference Glajchen and Bookbinder83), demonstrating that structured patient training can improve both perceived and actual (glycemic) control (Reference Barengo, Debussche and Besançon84;Reference Howorka, Pumprla and Wagner-Nosiska85), and that knowledge can be promoted by interactive features (Reference Cook, Levinson and Garside86). However, it is also possible that educational content might not just promote but also reduce perceived competence, for example, when a person is educated on something too complex to understand. Extant research is unclear whether structuring self-monitoring elements can enhance perceived self-management competence, yet shows potential improvements in self-determination (Reference Barengo, Debussche and Besançon84;Reference Howorka, Pumprla and Wagner-Nosiska85) (perceived control is used synonymously with the empowerment indicator self-determination/choice) (Reference Gutschoven and van den Bulck18).
Prior studies confirmed that graphic elements influence perceived impact and health-related behavioral intentions (Reference Villanti, Cantrell, Pearson, Vallone and Rath87), as well as data interpretation (Reference Jansen, McCaffery, Hayen, Ma and Reddel88). Our sample reiterated existing research, finding that analytical and graphic output features frequently went along with self-monitoring features in the sample (assigned to perceived impact of a diabetes app use).
The literature is quite established regarding features supporting indicators of behavioral empowerment, with the relevance of both HCP and social network support having been proven for diabetes self-management (Reference Rosland, Kieffer and Israel31;Reference Whitehead, Jacob, Towell, Abu-Qamar and Cole-Heath37;Reference Ramirez and Turner89). Research hints to the fact that mobile health applications are not efficient as stand-alone means for self-management support, but have to be included into the HCP–patient relationship to guarantee long-term use and effectiveness (Reference Katz, Mesfin and Barr90). Apps need to be officially approved to facilitate inclusion into diabetes care programs (e.g., government promotion) (Reference Kwong91). Inclusion of apps in diabetes programs provides synergetic effects, with a stronger obligation for app use, facilitated app selection and informed app use, facilitated (technology supported) HCP–patient cooperation, time-saving feedback procedures, improved data monitoring, and improved patient data collection (Reference Miller, Ziegler, Greenberg, Patel and Carter92). Moreover, diabetes apps, having been proven effective for self-management motivation (Reference Oh and Lee29;Reference Maki, O'Mally, Sekalala and Niezgoda93), should provide features to enable support by private patient networks; however, noting privacy issues involving personal medical records.
This analysis led to certain interpretive conclusions on the likelihood of maintained long-term use of the provided diabetes apps (app “stickiness” Reference Furner, Racherla and Babb94). The feature analysis revealed few strategies proven effective (reward systems, gaming elements, or entertainment features in less than 10 percent of apps) (Reference DeShazo, Harris, Turner and Pratt95) used by app providers to gain sustained users. Further obstacles that hindered sustained app use, such as frequent technical app failure (Table 1), required in-app purchases (31 percent), frequent advertising (25 percent; Table 2) and an unstable market dynamic (apps being removed from stores), prevented the apps from being viewed as reliable and trustworthy tools for diabetes self-management.
Overall, active and evidence-based strategies to motivate diabetics to sustain app use over time (Reference Thabrew, Stasiak, Garcia-Hoyos and Merry78) and more sophisticated mobile tools within the context of the HCP–patient relationship should be advanced (Reference Brahmbhatt, Niakan, Saha and Lau96). However, advanced diabetes apps cannot be designed by simply including multiple features to satisfy all theoretical concepts for all target audiences, leading to a laundry list of must-have app features. From a technical perspective this is not feasible or practical, with multiple features including social networking and gaming making a diabetes self-management app unwieldy and confusing for less tech-savvy target groups. Thus, instead of including all required features at once, segmenting, targeting, and positioning strategies can selectively improve diabetes app. Features have to be thoughtfully selected for specific target audiences, or target audiences have to be given choices in feature selection. App series or app “packages” provide an opportunity, offering several apps that can be individually selected and combined by the diabetic user (e.g., MySugr app series). However, the idea of combined apps has to be taken one step further with giving a choice to patients in selecting specific features relevant to them, to enhance usage and effectiveness of diabetes apps for self-management.
Study Limitations
Although this study situated empowerment as a predictor of self-management behaviors, we acknowledge that empowerment can be conceptualized as a process rather than a state (Reference Rappaport22), and further conceptual refinement is needed. The process perspective suggests that an initial level of patient empowerment continuously changes, and develops during the course of a patient's self-management process.
There was some concern about reliability and comparability of coding in the app analysis due to the dynamic nature of the diabetes apps and the marketplace. Accessibility to app features and functions, as well as displayed content, varied considerably. Acceptable inter-coder reliability was arrived at by means of appropriate strategies. Similarly, the fluctuating app market created stability problems for app selection (4 percent of apps had been removed from the app store between the app collection and the main coding); this needs to be addressed in future research.
The inclusion criteria potentially created bias by only examining free-to-download apps for diabetes self-management. For example, free-to-download apps may not be designed to support behavioral empowerment, because allowing shared decision making with HCPs would require virtual private network capabilities due to confidentiality issues and data protection. Further research should evaluate whether pay-to-download apps are more theoretically relevant than free-to-download apps. Similarly, including the top fifty apps in app stores might create bias in the evaluation. While it is likely that apps frequently downloaded appear at the top of the stores, this is an assumption, because ranking algorithms were nontransparent especially for the Apple App Store.
In conclusion, the study revealed that the diabetes self-management apps field is at a nascent stage of development, with current implementation failing to live up to the potential. Only a narrow set of app features supported psychological and behavioral empowerment in the sample, despite literature pointing toward the relevance of these features for empowerment. Further research is needed that examines app features corresponding to empowerment dimensions in greater detail, as well as what empowerment means for different users of apps.
Future research should further examine machine-based empowerment delivered through algorithms. Machine-based empowerment is not included in extant definitions of empowerment. It is worth contemplating whether behavioral empowerment can also be delivered by apps using automatic algorithms. In this regard, we can consider in what sense empowerment by a machine is still “behavioral” (being currently understood as influence from another individual).
The feature analysis revealed a scarcity of implemented strategies promoting app use and “stickiness.” Results suggested low app quality (technical failures, frequent advertising, lacking features); hence, further research is needed that combines aspects of app quality with the analysis looking into empowerment. It can be expected that app quality enhances or hinders the potential of apps for empowerment, by influencing its use (Reference Dutta, Pfister and Kosmoski97;Reference Inukollu, Keshamon, Kang and Inukollu98).
Conflicts of interest
None.






