Purpose
Although several guidelines for priority setting and health technology assessment (HTA) exist, many focus on the methodological aspect of HTA with little exploration of the facilitators and barriers beyond “contextual nuance.” Our study aims to identify and codify the facilitators and barriers to help implementing partners worldwide successfully institutionalize HTA and navigate complex systems for health-related policy making.
Introduction
HTA is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its life cycle. The purpose is to inform decision making to promote an equitable, efficient, and high-quality health system (Reference O'Rourke, Oortwijn and Schuller1). Although implementing HTA with transparent processes and products may take some time, a cohesive and comprehensive strategy is crucial to increase the chances of success of HTA administration (Reference Wild, Stricka and Patera2).
The International Network of Agencies for Health Technology Assessment (INAHTA) highlighted top ten challenges for HTA: scarcity of human resources; stakeholder involvement; the need to improve the existing HTA; inadequate data management; fragmented health system and shifting political context; enlarge the scope of HTA; increase the impact and influence of HTA; increase the demand for HTA; translate HTA into policy; and insufficient financial resourcing of HTA (Reference O'Rourke, Werkö, Merlin, Huang and Schuller3). An analysis of country-specific factors, the effects of international factors, and empirical findings need to be considered along with political aspects for HTA to be successfully adopted as a policy. Implementing partners need to understand the elements and challenges of HTA institutionalization to better “accelerate” within the dynamics and move toward universal health coverage (UHC).
Universal, quality, and equitable health services are the core principles of UHC to prevent financial hardship (4). However, countries worldwide face significant challenges in realizing this commitment, such as limited capacity and insufficient program planning, monitoring, and evaluation. Furthermore, weighing governments’, donors’, and implementing partners’ competing interests can be challenging for low- and middle-income countries (LMICs) (Reference Glassman, Chalkidou, Giedion, Teerawattananon, Tunis and Bump5). As countries move toward achieving UHC, the need for effective management and allocation of resources increases as the demand for better healthcare services grows.
Many guidelines are available for priority setting and HTA (Reference Wild, Stricka and Patera2;Reference Noorani, Husereau, Boudreau and Skidmore6;Reference Jansen, Helderman, Boer and Baltussen7). Nonetheless, these guidelines focus primarily on the technical aspects of conducting evaluations and contain limited information about navigating the political process of institutionalizing HTA. This systematic review aims to summarize the existing body of literature surrounding the facilitators and barriers in introducing HTA. The results of this global review synthesize documented lessons to inform countries, especially LMICs, about creating or strengthening their HTA institutions.
Methods
We searched six databases—Medline Complete, Health Policy Reference Center, Embase, Cochrane, Cumulative Index of Nursing and Allied Health Literature (CINAHL), and National Health Service Economic Evaluation Database (NHS EED) database—as well as gray literature from other relevant organizations for articles examining HTA programs globally. We included all English, French, and Spanish articles published on or before 31 December 2019. We used the keywords “health technology assessment,” “barrier,” and “facilitator,” as well as their synonyms, as a guide for capturing articles. The detailed list of synonyms and subject headings is presented in Supplementary Table 1. Given our focus on exploring facilitators and barriers to HTA introduction, we excluded articles focusing on the scope of HTA agencies and articles focusing on specific services or interventions, drugs, and technologies.
We removed duplicates, screened the titles and abstracts, and conducted a full-text review based on predetermined evaluation criteria adapted from Castro 2016 (Reference Jaramillo, Moreno-Mattar and Osorio-Cuevas8) and Rajan 2011 (Reference Rajan, gutierrez-ibarluzea and Moharra9) to encompass twenty-seven items included in four primary areas of interest: (1) barriers/facilitators, (2) motivations, (3) guidelines, and (4) institutional frameworks. After primary screening (by CG, MM, and AAC), results were extracted (by AAC, MM, and RK) and codified according to publication year and title, research questions answered, target users, and scope and whether competing interests were discussed. Ineligible articles were grouped to ease reporting and interpretation. Major points from uncategorized articles and third opinions were also considered. We used the top ten motives, enablers, and barriers for HTA promotion for high-income and low- and middle-income countries (Reference Rajan, gutierrez-ibarluzea and Moharra9) to categorize motivations for conducting HTA. Paper reviews were conducted independently by two reviewers, following the same data extraction template that was created prior to the review process. In case of disagreement, a third reviewer (HC) served as an arbitrator. Furthermore, we analyzed ten “drivers” that emerged with the ability to help or hinder HTA development (Reference Jaramillo, Moreno-Mattar and Osorio-Cuevas8) as guides for capturing articles. Articles identified were systematically examined using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) item checklist. This checklist is used to improve transparency in systematic reviews. These items cover all aspects of the manuscript, including title, abstract, introduction, methods, results, discussion, and funding. Furthermore, we used the PRISMA flow diagram to visually depict the flow of studies through each phase of the review process. It maps out the number of records identified, included, and excluded and the reasons for exclusions. All authors participated in synthesizing the findings presented in this paper.
Figure 1 shows the PRISMA flow diagram of this systematic review.
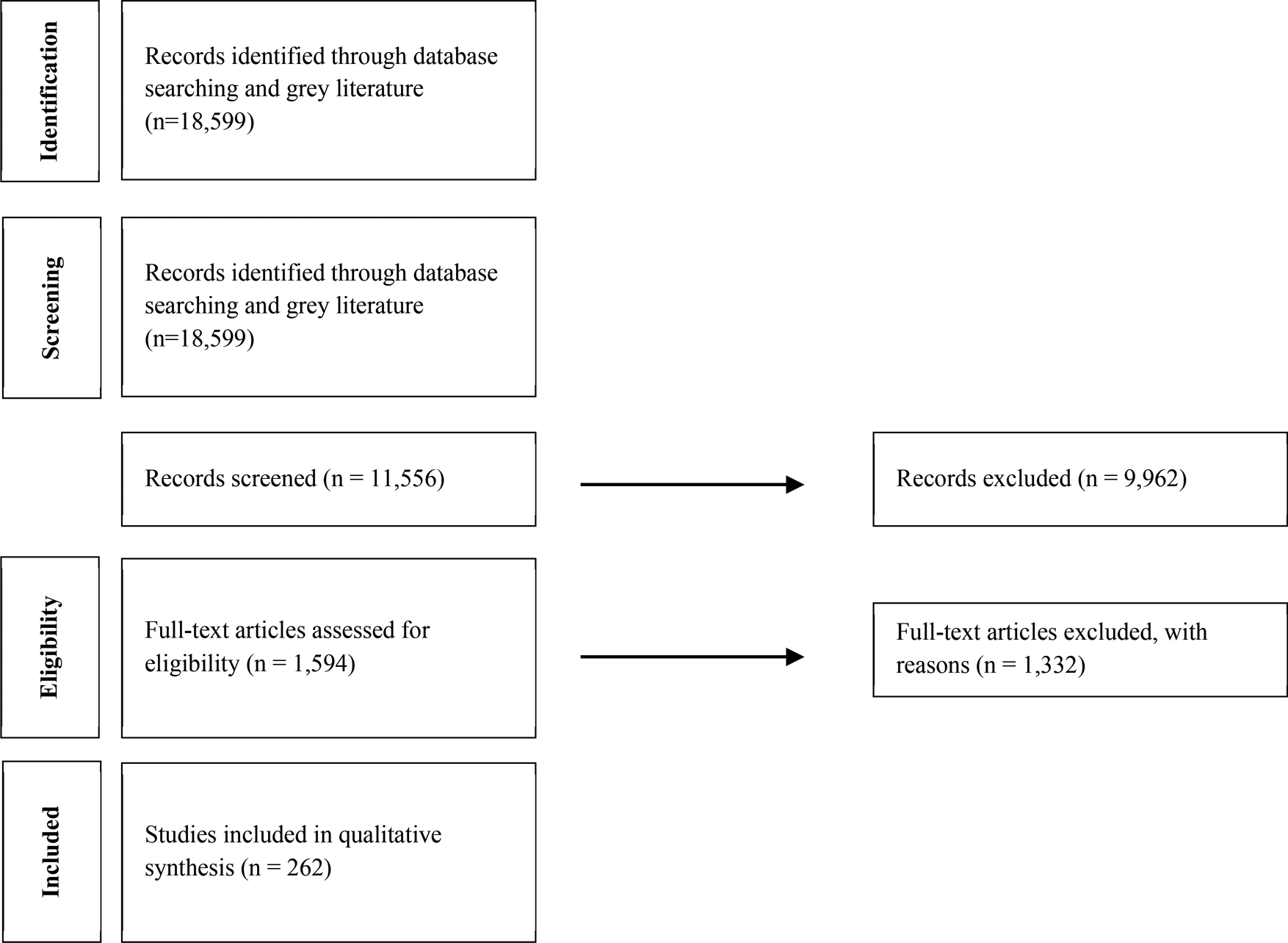
Figure 1. PRISMA flow diagram of included studies.
Results
During the initial process of the systematic literature review, 18,599 records were identified through the database searches. Of the 18,599 records, 11,556 from all over the world were eligible for the title and abstract review. A total of 9,962 records were deemed irrelevant to the scope and excluded after reviewing the title and abstract, leading to 1,594 articles for a full-text review. A total of 262 studies were included in the final synthesis, and data were codified and extracted. Table 1 presents the results of our data extraction.
Table 1. Evaluation criteria
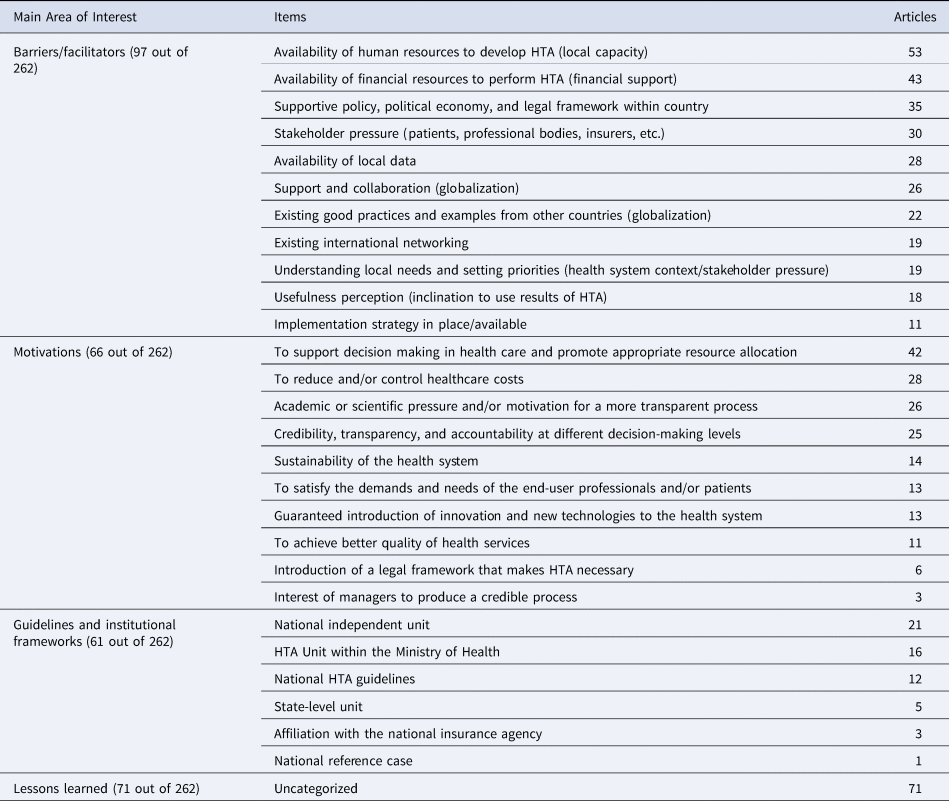
HTA, health technology assessment.
Ninety-seven articles discussed barriers/facilitators in implementing HTA. Of these, we observed a large number of articles that discussed local capacity (fifty-three articles), with emphasis on the lack of availability in human resources to build capacity as a barrier to develop HTA, particularly among LMICs. Nonavailability of financial resources was also listed as one of the main barriers for the implementation of HTA in low-income settings. Another important barrier for implementing HTA is nonavailability of local data, which was mentioned in twenty-eight articles. Alternatively, only eleven articles discussed the available implementation strategy in place. Although most of these articles discussed strategies in place for high-income countries such as Canada and the UK (Reference Martin, Polisena, Dendukuri, Rhainds and Sampietro-Colom15), a few of them also mentioned HTA implementation strategies in countries such as Colombia and Brazil (Reference Jaramillo, Moreno-Mattar and Osorio-Cuevas8).
Sixty-six articles discussed the motivation for implementing HTA. It was observed that one of the main motivators for HTA implementation is to support the decision-making process and promote appropriate resource allocation, which was discussed in forty-two of the sixty-six articles. The second most frequent motivator found in the literature is health-related cost reduction, discussed in twenty-eight articles. Credibility, transparency, and accountability were also listed as important motivators for HTA implementation, as they were mentioned in twenty-five articles. Interestingly, academic and scientific pressure for a more transparent process was also highly ranked as a motivator for HTA implementation and mentioned in twenty-five articles. Furthermore, six articles discussed the legal framework that motivates need, where three articles captured the managers’ interest in producing a credible process.
In terms of guidelines and institutional frameworks, we identified twenty-one articles that referred to HTA as an independent national unit and sixteen that discussed their HTA unit as a unit within the Ministry of Health (MOH). High-income countries such as Italy, the United Kingdom, Australia, Israel, and Germany have an independent national unit of HTA (Reference Ciani, Tarricone and Torbica10–Reference Fricke and Dauben14), whereas Hungary, Canada, Iran, Greece, and Singapore have established an HTA unit within MOH (Reference Martin, Polisena, Dendukuri, Rhainds and Sampietro-Colom15–Reference Pwee19). Furthermore, five articles discussed HTA units at the state level, and three articles discussed HTA institutional frameworks as an affiliate of the national insurance agency. Twelve articles discussed the national HTA guideline, whereas only one discussed a national reference case.
We also identified the lessons learned from seventy-one articles, although these lessons were not categorized, that cover critical concepts for implementing partners. For instance, an article from (Reference Marsh, Thokala, Youngkong and Chalkidou20) about the PRISMA project in West Java stated: “The project not only helped to define and reach consensus on a set of indicators on which evidence is required before decisions are taken, but it has also developed a mechanism through which various district-level stakeholders get the opportunity to be part of the decision-making process.” The context of this article is crucial to give an overview of the effect of lower-level stakeholder involvement in the HTA decision-making process. Overall, HTA is perceived as a very significant element of future health policy, as mentioned by Ahern et al. This is applicable to several articles that discussed the importance of HTA in the future of decision making for health care.
Details about each qualitative lesson learned, as well as the list of articles assessed by the evaluation criteria presented in Table 1, are available in Supplementary Tables 2–5.
Discussion
We reviewed a large number of internationally published articles (18,599) discussing priority setting and HTA. For barriers and facilitators, our results show that human resources, financial resources, a lack of local data, and political commitment are the primary barriers to introducing HTA. Informed by the context within which HTA operates, and from the manuscripts we reviewed, the authors opted to unify the analysis of barriers and facilitators together. This is because some aspects that are considered as barriers could be facilitators of change in other settings. For instance, Iran, Greece, and Singapore have successfully facilitated HTA institutionalization through an HTA unit within the MOH considering their existing policy and sufficient financing for HTA (Reference Martin, Polisena, Dendukuri, Rhainds and Sampietro-Colom15–Reference Yazdizadeh, Shahmoradi, Majdzadeh, Doaee, Bazyar and Souresrafil17). On the other hand, considering the fragmented healthcare system in the UK, Germany, and some other countries, having an HTA unit within the MOH would be the primary barrier to introducing HTA—they need to have an independent HTA unit to better institutionalize HTA into their healthcare system and policy (Reference Jaramillo, Moreno-Mattar and Osorio-Cuevas8;Reference Owen-Smith, Coast and Donovan11;Reference Tamir, Shemer, Shani, Vaknin and Siebzehner13).
Our findings align with those of research identifying a lack of resources, both financial and human, as challenges to introducing HTA, alongside the absence of government-regulated HTA (Reference Babigumira, Jenny, Bartlein, Stergachis and Garrison21). On the one hand, effective governance, collaboration, and cooperation among key stakeholders of the healthcare system were suggested as possible ways forward for the institutionalization of HTA (Reference Mueller22). On the other hand, the perceived usefulness of HTA is an important concept that has not received much coverage. Findings from HTA-related organization(s) suggest that they play an advisory role in most countries’ policy decisions. In terms of barriers to using HTA for decision making, it is suggested that raising awareness about the importance of HTA would help improve the incorporation of HTA findings in decision making (23). Justification of final recommendations is fundamental to utilization-focused evaluation. When agencies, communities, and other stakeholders agree that the recommendations of HTA reports are justified and based on solid evidence, they will be more likely to use the evaluation results for program improvement (24). Buy-in of stakeholders and implementing partners, who have an essential role in decision making as opinion leaders, is essential to realize the possibilities of policies related to novel health technologies.
We see cost control and resource allocation as the top two motivators, followed by promoting scientific and transparent processes. Research has shown that motivation (willingness) for HTA improves the chances for success (Reference Jeamu, Kim and Lee25). HTA offers clear advantages to decision makers, including evaluating the effects of a technology on health, distribution of resources, and other aspects of health system performance such as equity and responsiveness (Reference Velasco-Garrido and Busse26). There is a risk that HTA-related initiatives will not be impactful unless policy makers also examine the decision-making contexts within which HTA can be successfully implemented (Reference Gallego, Gool and Kelleher27).
A country's institutional framework will determine essential processes that need to be followed to ease the introduction of HTA. We found that the majority of HTA agencies around the world are framed as either independent national units or units within the MOH. The HTA department is intended to develop HTA guidelines and provide recommendations to the MOH through an accountable and transparent process (Reference Sastroasmoro28). Advice about accountable evidence-based decision making through a politically driven process is crucial to successfully institutionalize HTA. Institutionalization of health technologies and prioritization of interventions become crucial as countries move forward to deliver universal health coverage. Countries face complex choices about how to allocate their finite health budgets to meet the health priorities of their populations and how to choose from the vast array of technologies and interventions on offer (29). By prioritizing HTA through an independent organization, countries worldwide can further improve their resource allocation for health and increase public trust.
Existing systematic reviews about priority setting and HTA focus primarily on methods to evaluate health technology, practical approaches, and ethical aspects (Reference Noorani, Husereau, Boudreau and Skidmore6;Reference Schröder-Bäck, Thijssen, Peixoto and Evers30;Reference Oortwijn, Vondeling, Barneveld, Vugt and Bouter31). To the best of our knowledge, our review is the first to provide a clear picture for implementing agencies of barriers, facilitators, motivation, and guidelines to systematic priority setting, moving beyond key informant interviews and gray literature reports. This will help LMICs and high-income countries (HICs) whose HTA mechanisms are not well institutionalized.
We call on global researchers to develop a road map to HTA institutionalization for LMICs to cover critical aspects of HTA institutionalization that have not yet been widely covered. Within barriers/facilitators, we see that eighteen and eleven articles, respectively, covered usefulness perception and implementation strategy in place. Similarly, in motivation, the introduction of a legal framework and relevant stakeholders’ interest in producing a credible process did not receive much coverage, with only six and three articles each on these topics. As for guidelines and institutional frameworks, six articles covered affiliation with national insurance agencies, whereas only one article discussed the national reference case framework of HTA. The existence of a legal and regulatory framework conducive for implementation, fairness/ethics, and political considerations was infrequently reported (Reference Kaur, Prinja, Lakshmi, Downey, Sharma and Teerawattananon32). For instance, we found that recent guidelines from Philippines and Indonesia were heavily focused on the assessment piece (33;34). In addition, the International Decision Support Initiative (iDSI) put together a reference case but limited material on thinking about the details of who structures interventions for appraisal (Reference Wilkinson, Sculpher, Claxton, Revill, Briggs and Cairns35;Reference Woods, Revill, Sculpher and Claxton36). Countries need to find and develop an effective approach to HTA and coordinate efforts in the health sector (Reference Oortwijn, Vondeling, Barneveld, Vugt and Bouter31). Better coordination and motivation, as promoted by multisectoral approaches, might be among the best options to sustainably and ethically integrate HTA interventions into the country's health system (Reference Druetz37).
Future research or road maps should also discuss the quality and evaluation of HTA. Conflicting claims about a program's quality, value, or importance often indicate that stakeholders use different program standards or values in making their judgments. This type of disagreement can prompt stakeholders to clarify their values and reach a consensus on how the program should be judged (24).
Conclusion
This systematic review unpacks the dynamic and relevant contexts for understanding the HTA institutionalization process and provides a data set as a basis for future research. We discuss the most frequent and relevant barriers, facilitators, and motivations from around the world. We also use these experiences as a call for future research and development to support HTA institutionalization, particularly in LMICs and HICs whose HTA mechanisms are not well institutionalized. This review will help policy makers and practitioners learn from others to achieve tangible progress in confronting the most critical issues facing priority setting and HTA institutionalization. We also hope that this review helps implementation partners navigate the complexity of the political process and the various sectors’ blurred roles in practice. Finally, we hope to support countries to take their first step toward an accountable and transparent priority-setting process for better health resource allocation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0266462321000623.
Acknowledgments
The authors thank Barbara Timmons for her support in editing this manuscript.
Funding
This systematic review is partly made possible by the generous support of the American people through the US Agency for International Development (USAID) contract no. 7200AA18C00074. The contents are the responsibility of the authors (Management Sciences for Health) and do not necessarily reflect the views of the USAID or the United States Government.
Conflict of Interest
The authors declare no conflict of interest.




