Introduction
The Chicxulub impact is considered one of the most devastating episodes in the recent Earth's history (Alvarez et al., Reference Alvarez, Alvarez, Asaro and Michel1980). The event that took place at the Cretaceous–Paleogene boundary caused a global catastrophe on Earth. Due to the impact, a large cloud of ashes and dust was released to the atmosphere, shielding the solar radiation from reaching the surface of the planet (Pope et al., Reference Pope, Baines, Ocampo and Ivanov1994; Pierazzo et al., Reference Pierazzo, Hahmann and Sloan2003). Cold and darkness covered the surface of Earth during several years after the impact.
The biosphere was particularly affected during this episode. As a consequence of darkness, photosynthesis collapsed during the first 2 years. More than 75% of the species became extinct, including the end of the dinosaur's reign (Alvarez et al., Reference Alvarez, Alvarez, Asaro and Michel1980). The structure of the biosphere was also sensibly altered after the impact. While the biggest organisms practically disappeared, others like detritivores and mushrooms were remarkably favoured during this period, due to the large amount of organic matter and cadavers of dead animals (Pope et al., Reference Pope, Baines, Ocampo and Ivanov1994; Vellekoop et al., Reference Vellekoop, Sluijs, Smit, Schouten, Weijers, Sinninghe Damsté and Brinkhuis2014).
During the present work, we continue exploring the potential influences on the biosphere induced by an impact like Chicxulub's. However, in this particular case, we discuss a practically unexplored issue in the literature. How would a mass impact like Chicxulub's affect the chemosynthesis done by anammox communities? What implications would these affectations bring to the biosphere, or how do they affect the dynamics of our planet at global scale. The potential answers to these questions are discussed in the next sections.
Anammox communities: peculiarities and transcendence
For a long time, chemosynthetic (chemoautotrophic) organisms were considered species confined or restricted to very small habitats. Before 1990, most of the chemosynthetic species were found in rather extreme habitats, such as submerged caves or in the surrounding of fumaroles and hydrothermal vents. Accordingly, opposite to the role conferred to photosynthesis, chemosynthesis was considered a marginal process with little contribution to the biogeochemistry of our planet at global scale.
However, the discovery of anammox in 1995 completely changed this picture (Mulder et al., Reference Mulder, van de Graaf, Robertson and Kuenen1995). With an ample variety of species, anammox appears as one of the most extended organisms in our planet (Dalsgaard et al., Reference Dalsgaard, Thamdrup and Canfield2005). Showing an amazing capability of adapting to a wide range of environmental conditions, anammox habitats include the seas (Smith et al., Reference Smith, Böhlke, Song and Tobias2015), underground waters (Thamdrup et al., Reference Thamdrup, Dalsgaard, Jensen Marlene, Ulloa, Farías and Escribano2006), aquatic and terrestrial ecosystems (Xi et al., Reference Xi, Bai, Zhang and Fang2016). Furthermore, the discovery of anammox completed a missing piece in the global cycle of nitrogen (Devol, Reference Devol2003; Arrigo, Reference Arrigo2004), a fact that finally proved the role of these particular species of chemosynthetic organisms in the whole dynamics of our planet.
During this work, we estimated some potential affectations on these communities associated with the occurrence of a massive impact like Chicxulub's. Two different environmental perturbations were considered in our analysis. Firstly, we considered the influence on these communities of the rapid short-term global cooling following the Chicxulub impact as a consequence of large number of material (soot, dust, ashes, aerosols) ejected to the atmosphere. Secondly, we also estimated potential affectations associated with the process of ocean acidification that preceded the event, a process driven, basically, by the transit of many sulphur and nitrogen compounds from the atmosphere to the ocean. In both cases, the selection of the environmental variables responds, on one hand, to the availability of plausible estimations about the real impact of Chicxulub on the climate of the Earth; on the other hand, due to the high sensitivity reported in the literature for the anammox species under variations of temperature and pH.
The impact of global cooling
After the impact of Chicxulub, the thick layer of material expelled into the atmosphere caused a remarkable reduction in the levels of solar radiation on the surface of our planet. It is considered that the drastic luminous attenuation was mainly due to the large amount of soot and sulphates injected into the atmosphere during the impact (Bardeen et al., Reference Bardeen, Garcia, Toon and Conley2017; Kaiho and Oshima, Reference Kaiho and Oshima2017). As a result, the surface temperature dropped rapidly, resulting in a pronounced global winter (Bardeen et al., Reference Bardeen, Garcia, Toon and Conley2017). Even though there is a certain level of uncertainty in these estimations, it is very probable that the temperature dropped by about 20°C (Pierazzo et al., Reference Pierazzo, Hahmann and Sloan2003), being able to reach even greater drops in correspondence with the results derived in more recent studies (Artemieva and Morgan, Reference Artemieva and Morgan2017; Bardeen et al., Reference Bardeen, Garcia, Toon and Conley2017). According to these new estimates, the impact winter phase could have been not only more drastic, but much more persistent, reaching from several years to decades.
The oceans were also affected because of global cooling. It is estimated that changes in temperature along the water column reached hundreds of metres, progressively attenuating with depth (Bardeen et al., Reference Bardeen, Garcia, Toon and Conley2017; Kaiho and Oshima, Reference Kaiho and Oshima2017). It is important to note that, although the estimated temperature decrease is much lower than those reported on the surface, its effect is much more persistent due to the enormous heat capacity of the water. Taking into account these elements, we explored the affectations on the relative activity of the anammox associated with the temperature variations that preceded a massive impact like Chicxulub's.
To make our estimates, we will consider the temporal evolution of the mean sea temperature based on the depth reported by Kaiho and Oshima (Reference Kaiho and Oshima2017) for two different impact scenarios. The first, of lower intensity, includes the emission of 1500 Tg of black carbon in the form of soot, while the second, of greater intensity, involves the emission of 2600 Tg of soot.
On the other hand, in order to quantify the direct effect of temperature on the anammox activities, we consider an Arrhenius type model (Lotti et al., Reference Lotti, Kleerebezem and Loosdrecht2015; Zhu et al., Reference Zhu, Li, Dong, Wang and Zhang2017; Hoekstra et al., Reference Hoekstra, de Weerd, Kleerebezem and van Loosdrecht2018). The activation energy was considered equal to Ea = 239 kJ mol−1 consistent with the value estimated by Hoekstra et al. (Reference Hoekstra, de Weerd, Kleerebezem and van Loosdrecht2018). This parameterization, in particular, allows us to estimate changes in anammox activity associated with long-term temperature fluctuations, a condition that makes it ideal for exploring a scenario such as ours. The results of applying this procedure for an event that emits 1500 Tg of soot into the atmosphere are shown in Fig. 1.

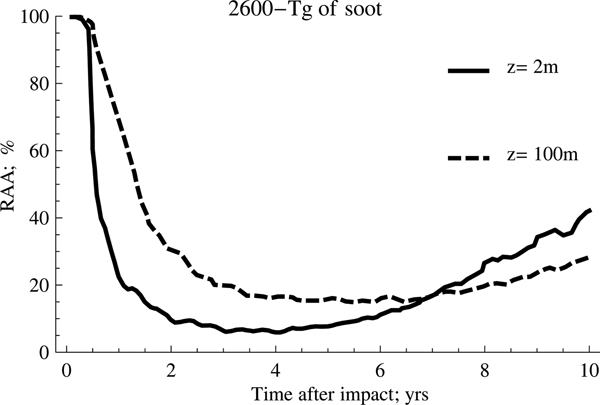
Fig. 1. Effect of temperature on anammox relative activity (RAA) for two different depths considering 1500 Tg of soot.
The results of applying the same procedure are shown in Fig. 2 but considering, in this particular case, that the level of soot emitted to the atmosphere reached 2600 Tg.
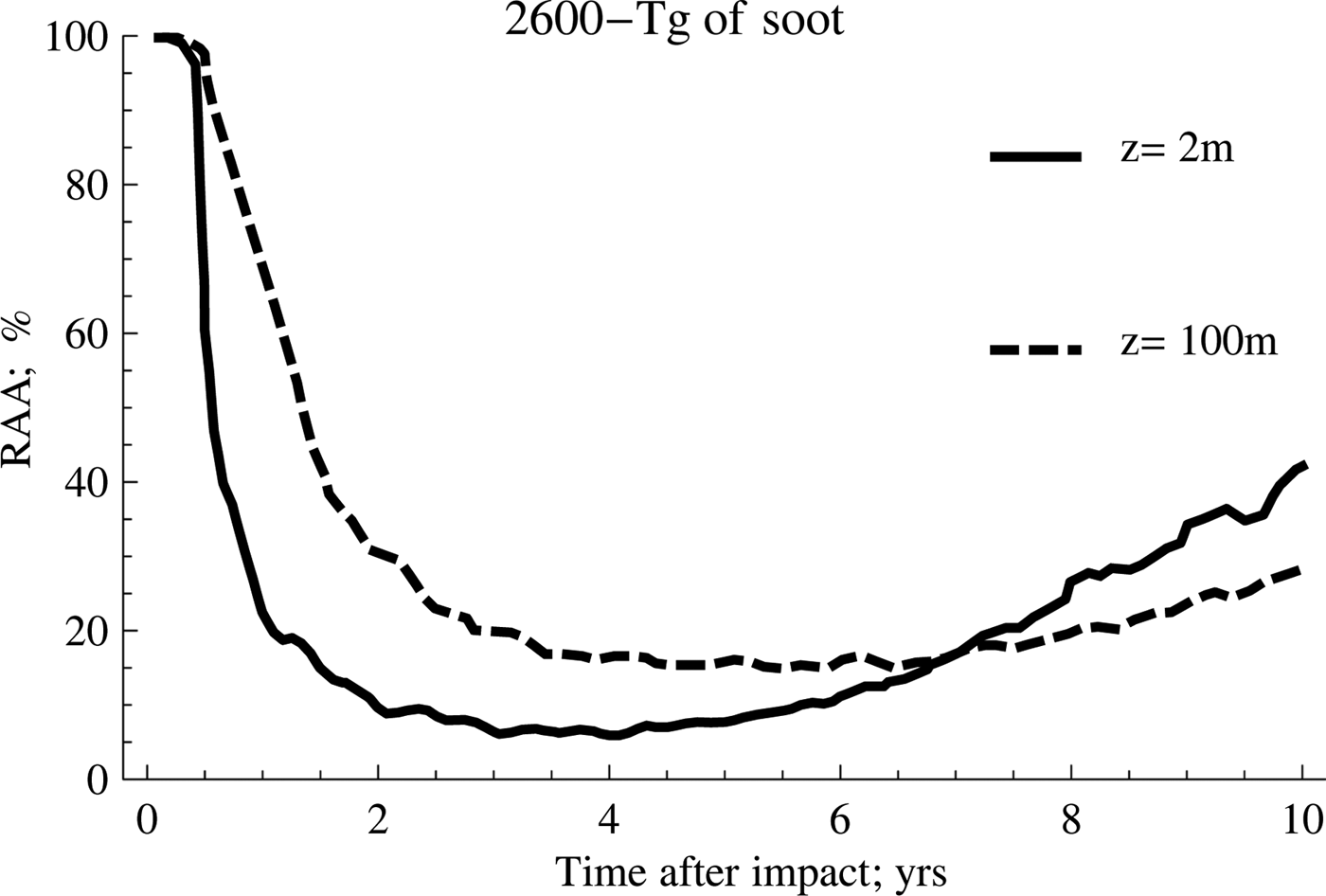
Fig. 2. Effect of temperature on anammox relative activity (RAA) for two different depths considering 2600 Tg of soot.
As can be seen from the previous figures, the anammox activities are considerably reduced for several years after the event. The activity will depend largely on the amount of soot emitted into the atmosphere and the ocean depth considered in the study, reaching, as a general trend, a significant decrease in the range from 2 to 4 years after the impact. It is also possible to appreciate clearly that the anammox species confined to deeper waters take longer to recover the original activity than those that inhabit shallower waters, despite registering a lower fluctuation of the average temperature.
On the other hand, the scenario with the highest amount of soot (2600 Tg) shows the highest inhibition rates and the highest persistence over time, a trend that is shown more clearly in Fig. 3.
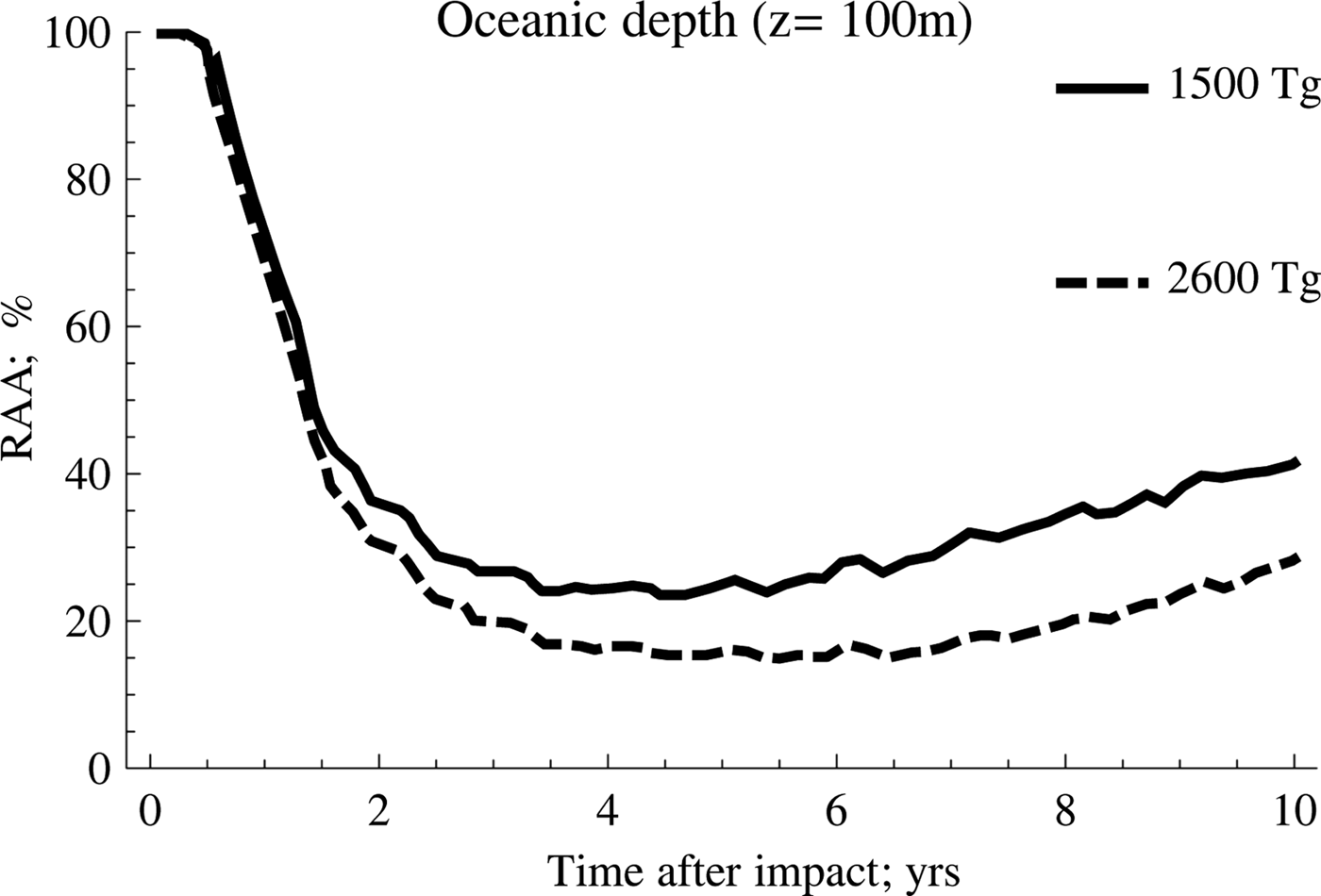
Fig. 3. A comparison between anammox relative activity (RAA) derived as a function of the amount of soot.
According to this, the behaviour for both cases is very similar during the first 2 years after the event occurred. However, as of this date, the recovery of the anammox tends to occur in a much more accentuated manner when the amount of soot is lower, which implies that the temperature of the water rises more rapidly. Meanwhile, the second case reflects the persistence of low temperatures for longer, a situation that tends to be quite unfavourable for the development of these particular kind of species.
The impact of acidification
Acidification is recognized as another of the most important collateral effects associated with the occurrence of a mass impact on Earth (Tyrrell et al., Reference Tyrrell, Merico and Armstrong McKay2015). The deposition of a large amount of chemical compounds, mainly in the form of aerosol sulphates, are responsible for a decrease of pH values affecting both the ocean and freshwater ecosystems. In the case of Chicxulub, this effect was particularly enhanced by the peculiarities of the target rock, mostly a mixture of carbonate- and sulphate-rich rocks (Pope et al., Reference Pope, Baines, Ocampo and Ivanov1994; Pierazzo et al., Reference Pierazzo, Hahmann and Sloan2003; Ohno et al., Reference Ohno, Kadono, Kurosawa, Hamura, Sakaiya, Shigemori, Hironaka, Sano, Watari, Otani, Matsui and Sugita2014). Other chemical species like carbon dioxide and nitrates may have contributed, but in a lower extent, to the acidification process in the ocean (Tyrrell et al., Reference Tyrrell, Merico and Armstrong McKay2015).
The drop of pH may have caused a noticeable impact in many communities of microorganisms. For instance, according to the results of Carvajal-Arroyo et al. (Reference Carvajal-Arroyo, Sun, Sierra-Alvarez and Field2013), there is a drastic inhibition of suspended anammox cultures if the pH of the medium is below 6.5 or above 8.0. According to that, and taking into account that many species of anammox show high sensitivity to pH variations, let us estimate how the productivity of these communities was affected by the fall of the typical pH values in the oceanic surface after the impact. As the severity of this process depends not only on the net amount of material deposited, but also on how fast this material is delivered to the ocean, two rather extreme scenarios were considered. In both cases, our analyses were based in the pH behaviour for sulphate additions reported previously by Tyrrell et al. (Reference Tyrrell, Merico and Armstrong McKay2015).
In the first case, we study the induced affectations considering 15, 30, 60 PMol of sulphates where the e-folding time for the deposition was set to 6 months. In the second case, the same procedure was repeated but the scale of time considered was remarkably reduced to only 10 h, in accordance with the more recent estimates suggested by Ohno et al. (Reference Ohno, Kadono, Kurosawa, Hamura, Sakaiya, Shigemori, Hironaka, Sano, Watari, Otani, Matsui and Sugita2014). As it is stressed by Tyrrell et al. (Reference Tyrrell, Merico and Armstrong McKay2015), such rapid additions exacerbate the influence in the pH values if we compare them with the behaviour displayed by the former case.
On the other hand, as a prototype of the pH influence on anammox communities, we considered the data published by Carvajal-Arroyo et al. (Reference Carvajal-Arroyo, Sun, Sierra-Alvarez and Field2013). In this case, data were taken directly from the plot and conveniently interpolated to facilitate computations. The results derived in each case are shown in Figs. 4 and 5.
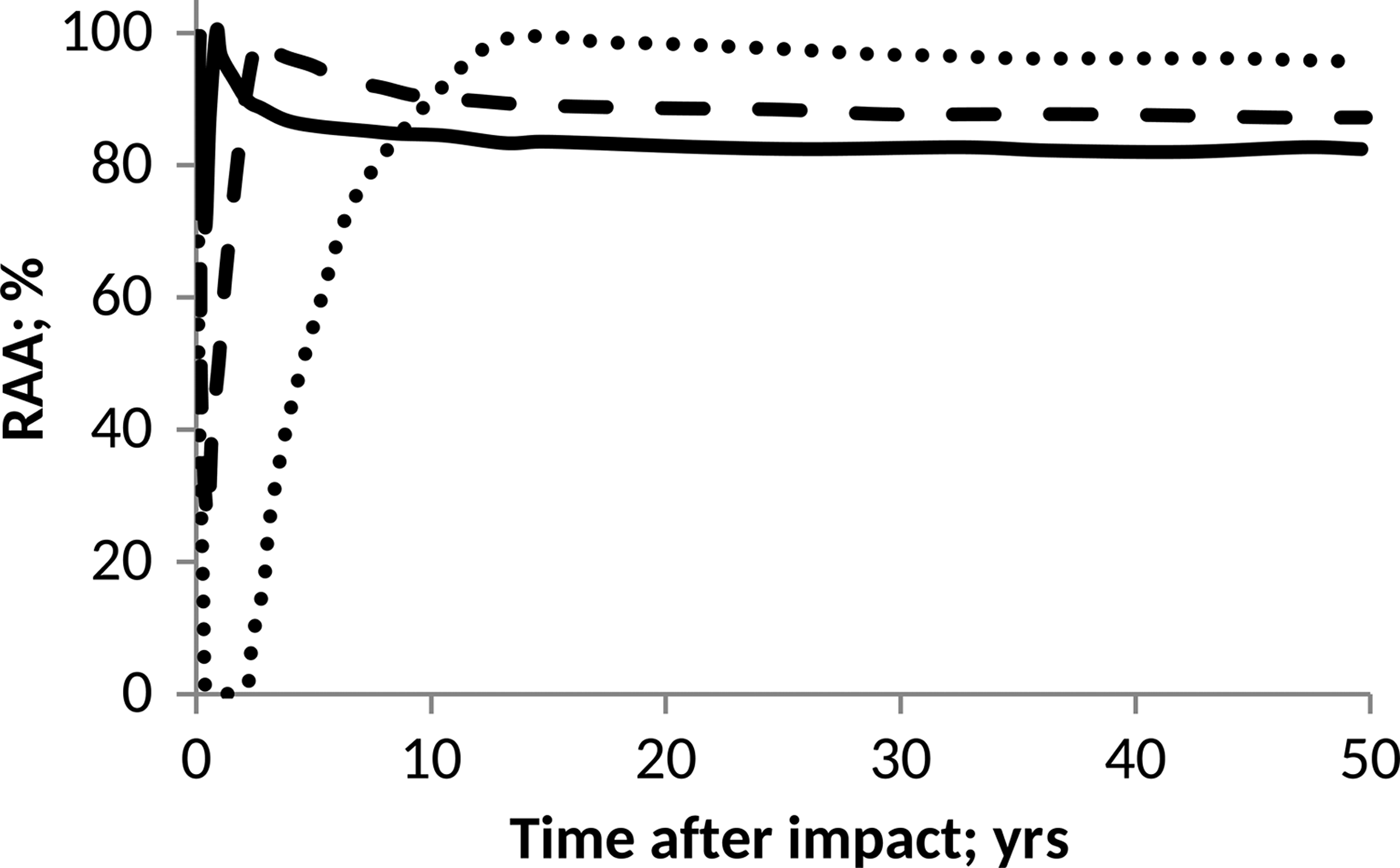
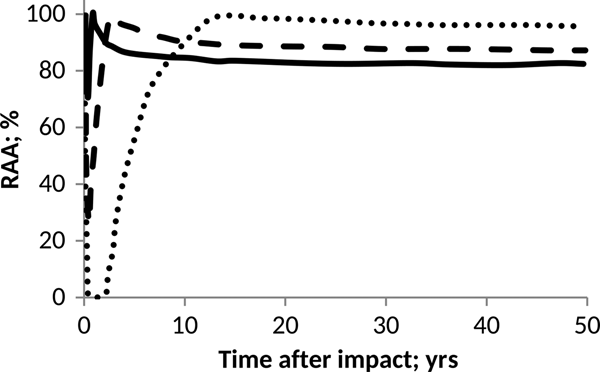
Fig. 4. Effect of pH on anammox relative activity (RAA) in suspended culture with e-folding time of 6 months. H2SO4 addition of 15 Pmol (solid), 30 Pmol (script) and 60 Pmol (round dot).
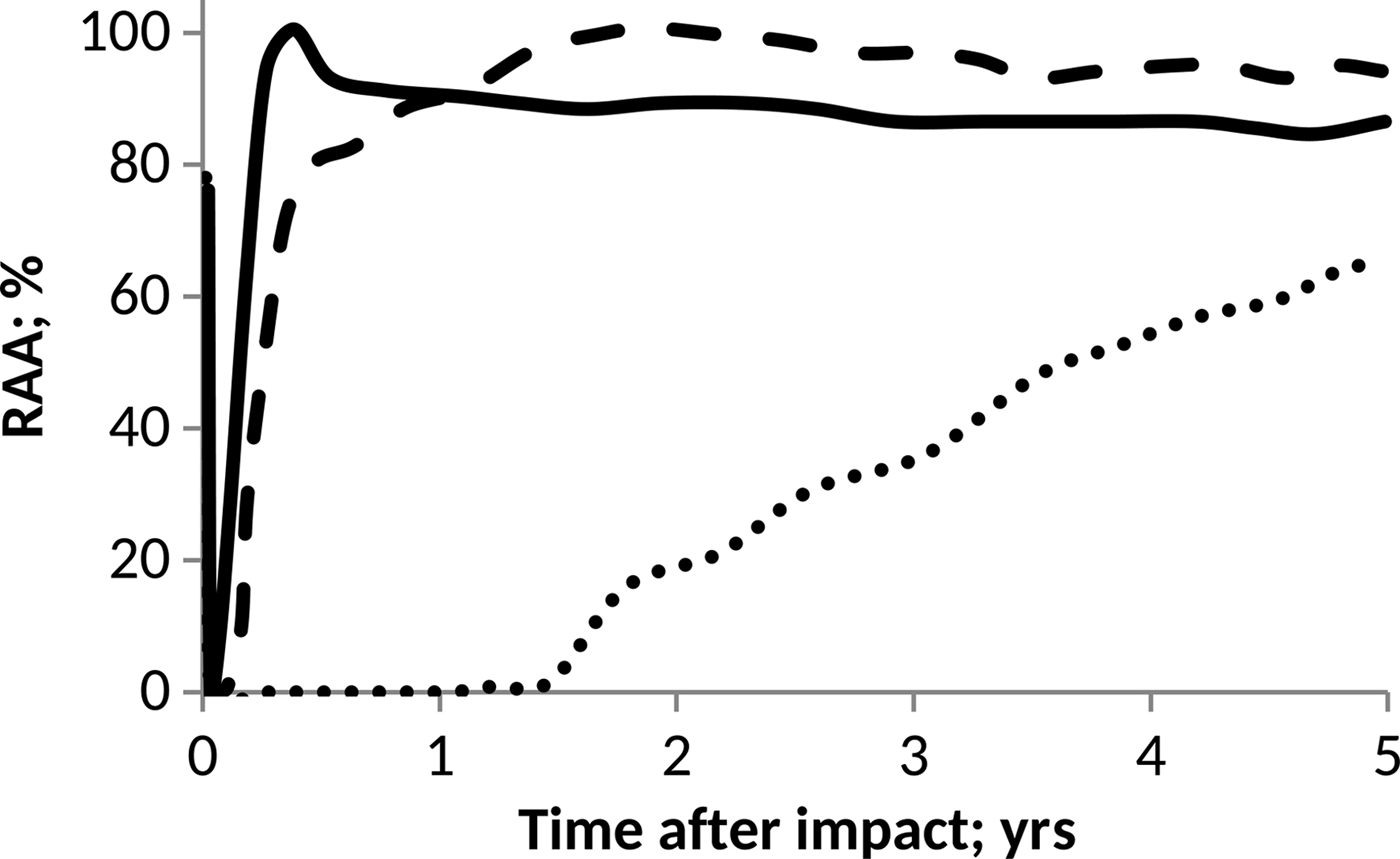
Fig. 5. Effect of pH on anammox relative activity (RAA) in suspended culture with e-folding time of 10 h. H2SO4 addition of 15 Pmol (solid line), 30 Pmol (script) and 60 Pmol (round dot).
According to Figs. 4 and 5, anammox communities could be seriously affected as a result of the decrease in pH values. The magnitude of such affectations can vary according to the net quantity of sulphate produced during the event and the timescale that governs the process of atmospheric deposition. The greater and faster are the additions of H2SO4, the more marked and abrupt are the changes in the pH of the ocean, and consequently, a more devastating and inhibitory effect on anammox bacteria is seen.
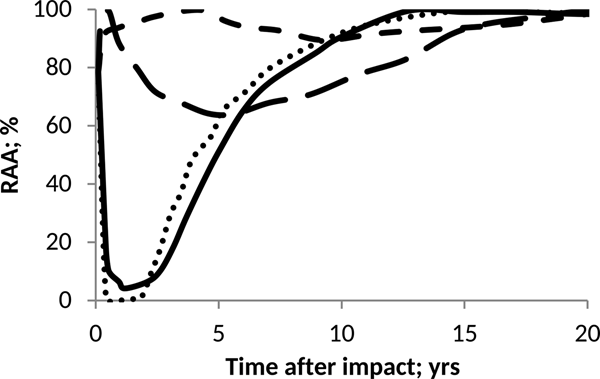
On the other hand, Fig. 6 shows that if the addition of H2SO4 is slower, the adverse effects on the relative activity of anammox will be less drastic but more lasting over time. The above corresponds to a longer acidification period caused by a low deposition rate of sulphuric acid on the ocean. It can be inferred from Fig. 6 that anammox shows good recovery capacities once the sea pH conditions return to normal.

Fig. 6. Effect of pH on anammox relative activity (RAA) in suspended culture for H2SO4 addition of 60 Pmol and different e-folding time: 0.5 year (round dot), 1 year (solid line), 5 years (large script) and 10 years (script).
In our analyses, we also considered the potential influence of nitrates on the annamox relative activity. We must take into account that ![]() ${\rm NO}_3^- $ has an inhibitory effect on anammox if its concentration is higher than 50 Mm (Carvajal-Arroyo et al., Reference Carvajal-Arroyo, Sun, Sierra-Alvarez and Field2013). However, according to our estimates, the influence of this particular chemical species was negligible on the RAA due to its low concentration in the ocean.
${\rm NO}_3^- $ has an inhibitory effect on anammox if its concentration is higher than 50 Mm (Carvajal-Arroyo et al., Reference Carvajal-Arroyo, Sun, Sierra-Alvarez and Field2013). However, according to our estimates, the influence of this particular chemical species was negligible on the RAA due to its low concentration in the ocean.
Extrapolating to freshwater and terrestrial ecosystems
Due to their much smaller volume, freshwater ecosystems would suffer more due to the drastic diminution of surface temperature, reaching up to 26°C in some regions (Bardeen et al., Reference Bardeen, Garcia, Toon and Conley2017). Due to a similar reason, freshwater ecosystems would be particularly affected by acidification during the Chicxulub impact. With a rather limited volume, the effects of dilution in this kind of system tend to be small. In addition, the entry of substances as a result of rain and evaporation could be more harmful for anammox bacteria and all bacterial communities living in these ecosystems than for those living in the ocean, therefore inhibitory effects would be more intense and lasting. Something similar is expected for the case of groundwater ecosystems.
Several atmospheric modelling of the K/Pg limit used atmospheric residence times of sulphur from several months to a few years (Pierazzo et al., Reference Pierazzo, Hahmann and Sloan2003). However, recent studies have proposed an alternative scenario, very different from the one generally accepted for the K/Pg event: it is suggested that immediately after the impact, most of the sulphate aerosols (sulphuric acid aerosols) could have been eliminated by large particles of silicates that fell rapidly back to Earth, delivering the load of H2SO4 in only one or few days (Ohno et al., Reference Ohno, Kadono, Kurosawa, Hamura, Sakaiya, Shigemori, Hironaka, Sano, Watari, Otani, Matsui and Sugita2014).
The net effect of decreasing temperature and pH: a global vision
According to the results obtained in the previous sections, communities of anammox could be seriously affected during a massive impact by the combined effect of temperature and pH. In both cases, the levels of inhibition could be significant considering the high sensitivity of these species to a combined decrease in temperature and pH. We should bear in mind that most of the anammox species so far studied show greater activity in warm conditions (25–30°) and neutral or slightly basic media (pH 7–8) (Tomaszewski et al., Reference Tomaszewski, Cema and Ziembińska-Buczyńska2017).
On the other hand, we must also consider that the anammox activity does not depend independently of both variables. In most of the cases studied (Daverey et al., Reference Daverey, Chei, Dutta and Lin2015; Tomaszewski et al., Reference Tomaszewski, Cema and Ziembińska-Buczyńska2017), a combination of this type (low temperatures and pH) can reinforce the negative effects on the anammox in a non-linear way, which suggests a significant increase in the degree of inhibition in a scenario similar to Chicxulub's.
Conclusions
According to this study, the process of global cooling and ocean acidification as a consequence of an asteroid or comet impact could significantly alter the activity of anammox bacteria and, by extension, other chemosynthetic communities whose metabolism is influenced by changes in temperature and pH. However, even when the influence of temperature and pH during this study is rather limited to the anammox suspended in the ocean surface, communities in the deep sea sediment could also be affected by the accumulation of ash and other sediments, a well-documented fact in the case of volcanic eruptions (Song et al., Reference Song, Buckner Caroline, Hembury Deborah, Mills Rachel and Palmer Martin2014). Since the inhibition increases as a function of the ash thickness, it is probable that in a catastrophic scenario like the Chicxulub impact, this effect could be significant. We must take into account that in marine sediments, anammox communities can reach 79% of N2 production (Dalsgaard et al., Reference Dalsgaard, Thamdrup and Canfield2005; Trimmer and Nicholls Joanna, Reference Trimmer and Nicholls Joanna2009).
On one hand, even when the affectations on the anammox communities at global scale may reduce the rate of N2 removal in appreciable way, the net impact on the nitrogen biogeochemical cycle remains unclear. Most of the effects considered in our analyses: global cooling, acidification, sulphate deposition combined with a noticeable change in the radiative regime in the surface of the planet, also affect the other components of the nitrogen cycle (Shi et al., Reference Shi, Kranz, Kim and Morel2012; Rees et al., Reference Rees, Turk-Kubo, Al-Moosawi, Alliouane, Gazeau, Hogan and Zehr2017). Probably, many mechanisms for nitrogen fixation were also affected with the reduction of sunlight and the drop of temperature at the surface of the planet. For instance, many nitrogen fixing microorganisms associated with land plants lost their potential during this period. On the other hand, the increases of organic matter from dead organisms favoured the increase of organic nitrogen dissolved in the ocean. In general, even without a full modelling, it can be expected that stagnation of the nitrogen cycle was the most probable condition on Earth, a situation that would persist several years after the impact.
Unravelling the dynamics of the Earth after a mass impact arises as a key element to understand the biogeochemical evolution of our planet. However, a better comprehension of the set of processes that followed an event of this nature implies the integration of photosynthesis and chemosynthesis during these catastrophic episodes. Such kind of modelling also implies a better understanding of the metabolic kinetics of the different microorganisms involved in this complex process (Kartal et al., Reference Kartal, Maalcke, de Almeida, Cirpus, Gloerich, Geerts, Op den Camp, Harhangi, Janssen-Megens, Francoijs, Stunnenberg, Keltjens, Jetten and Strous2011; Brunner et al., Reference Brunner, Contreras, Lehmann, Matantseva, Rollog, Kalvelage, Klockgether, Lavik, Jetten, Kartal and Kuypers2013).