Published online by Cambridge University Press: 18 March 2015
The origin of the enantiomeric excess found in the amino acids present in the organic matter of carbonaceous meteorites is still unclear. Selective adsorption of one of the two enantiomers existing after a racemic formation could be part of the answer. Hereafter we report a comparative study of the adsorption of the R and S enantiomers of α-alanine and lactic acid on the hydroxylated { $10\bar 10$} chiral surface of α-quartz using numerical simulation techniques. Structurally different adsorption sites were found with opposite R versus S selectivity for the same molecule–surface couple, raising the problem of whether to consider adsorption as a local property or as a global response characteristic of the whole surface. To deal with the second term of this alternative, a statistical approach was designed, based on the occurrence of each adsorption site whose energy was calculated using first principle periodic density functional theory. It was found that R-alanine and S-lactic acid are the enantiomers preferentially adsorbed, even if the adsorption process on the quartz {
$10\bar 10$} chiral surface of α-quartz using numerical simulation techniques. Structurally different adsorption sites were found with opposite R versus S selectivity for the same molecule–surface couple, raising the problem of whether to consider adsorption as a local property or as a global response characteristic of the whole surface. To deal with the second term of this alternative, a statistical approach was designed, based on the occurrence of each adsorption site whose energy was calculated using first principle periodic density functional theory. It was found that R-alanine and S-lactic acid are the enantiomers preferentially adsorbed, even if the adsorption process on the quartz {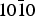 $10\bar 10$} surface stays with a disappointingly poor enantio-selectivity. Nevertheless, it highlighted the important point that considering adsorption as a global property changes perspectives in the search for more efficient enantio-selective supports and more generally changes the way to apprehend adsorption processes in astro-chemistry/biology.
$10\bar 10$} surface stays with a disappointingly poor enantio-selectivity. Nevertheless, it highlighted the important point that considering adsorption as a global property changes perspectives in the search for more efficient enantio-selective supports and more generally changes the way to apprehend adsorption processes in astro-chemistry/biology.