To the Editor—Diagnostic testing is essential in distinguishing patients who have a disease from those who do not. The accuracy of a test is described by sensitivity and specificity. Sensitivity reflects how many patients with disease have a positive test, and specificity reflects how many patients without disease have a negative test. When a test is applied to different populations, pretest probability changes, but not sensitivity or specificity because these are inherent characteristics of the test.Reference Iorio, Spencer and Falavigna1 However, clinical experience suggest that urine cultures and other tests do not have fixed testing characteristics and that specificity can and does vary by population. We sought to explore the rule that sensitivity and specificity are fixed using a commonly ordered test—the urine culture.
Laboratory values reflect underlying microbiology, physiology, or biochemistry and must be accurate and consistent, but to be clinically useful a diagnostic test must distinguish patients with and without clinical disease. Urinary tract infection (UTI) requires symptoms in addition to the presence of a microorganism. For microbiology tests, positive results indicating the presence of a potentially pathogenic organism in the absence of symptomatic disease are common and are known as asymptomatic colonization or asymptomatic bacteriuria (ASB). Treatment of ASB is a leading cause of antibiotic overuse.Reference Nicolle, Gupta and Bradley2 Urine cultures are among the most commonly ordered tests but are often misinterpreted, especially in older, sicker populations.
We searched the English-language medical literature using the terms urine culture, urinary tract infection, and sensitivity or specificity. This search was supplemented by a manual review of the bibliographies of all identified articles. One author (K.C.T) initially screened the titles and abstracts of the search results. Two authors (K.C.T and D.J.M.) then independently reviewed and abstracted data from the articles identified as relevant.
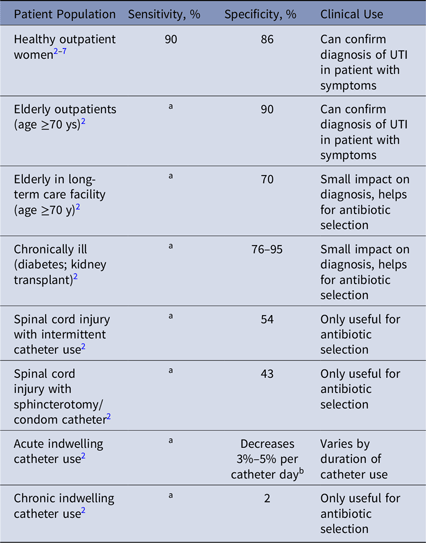
We identified 1,075 articles, of which 18 satisfied the criteria of reporting sensitivity or specificity of urine cultures.Reference Nicolle, Gupta and Bradley2–Reference Stamm, Counts, Running, Fihn, Turck and Holmes7 Of these, 13 were summarized in 1 guideline.Reference Nicolle, Gupta and Bradley2 Articles were analyzed for sensitivity and specificity using 105 colony-forming units per milliliter as the criteria for comparison (Table 1). For multiple studies of the same population, a weighted average for sensitivity and specificity was calculated.
Table 1. Sensitivity, Specificity and Clinical Usefulness of Urine Culture for Diagnosis of Urinary Tract Infection (UTI) in Different Patient Populations
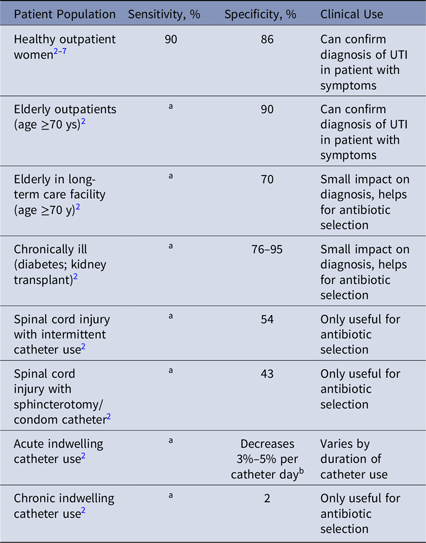
Note. For brevity, studies of specificity are summarized in the Infectious Disease Society of America guidelines for management of asymptomatic bacteriuria (ASB).Reference Nicolle, Gupta and Bradley2 These guidelines report false-positive rate or ASB, which is 1-specificity. When rates of ASB are described, this is the false positive rate of urine culture testing, which was calculated as 1-specificity of the test.
a No studies identified reported sensitivity of urine cultures in these populations.
b The prevalence of asymptomatic bacteriuria or a positive urine culture without UTI increases 3%–5% per day of catheterization, therefore the specificity decreases by 3%–5% per day.
Urine cultures had a sensitivity of ~90% for UTI in healthy outpatient women (Table 1). We found no studies of the sensitivity of urine cultures for other populations. We found highly variable specificity for urine culture to identify UTI, from 80%–90% in healthy outpatients down to nearly 0% in patients with chronic indwelling catheters.
The specificity of urine cultures varies greatly by population, from 0% to 90%, which contradicts the rule that the specificity of a diagnostic test does not change by population. This finding has broad implications for urine testing and treatment in different patient groups. Biologically, this variability in specificity results from different populations having factors that lead to chronic bacterial colonization, leading to false-positive results in patients without symptoms of UTI. Although urine cultures are accurate for presence of bacteria, they are nonspecific for the presence of clinical UTI. Other examples of widely used tests in which sensitivity or specificity vary by population are Clostridium difficile Reference Bagdasarian, Rao and Malani8 and B-type natriuretic peptide (BNP).Reference Taylor, Verbakel and Feakins9
Correct application of diagnostic testing is complex. Appreciating potential variation in specificity by population tested is important for patient care. With urine cultures, treatment of false-positive urine cultures, or ASB, risks antibiotic exposure without clinical benefit. Varying specificity for this test across populations emphasizes the need to identify sensitivity and specificity within similar populations to the ones that the test will be applied to, and potentially determine, sensitivity and specificity for multiple populations. In an age of electronic records, the reporting of urine cultures and the predictive value of the results could easily be tailored to the type of patient tested. Additionally, the varied specificity of urine cultures means we need different diagnostic stewardship for urine cultures in long-term care, inpatient care, and outpatient care settings.Reference Morgan, Malani and Diekema10 By appreciating the nuances of diagnostic testing for UTI and other diseases, we can leverage currently available technology to provide better diagnoses and patient care.
Acknowledgments
The participation of coauthor K.C.T should not be construed to represent the views or policies of the US Food and Drug Administration. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans’ Affairs or the US government.
Financial support
D.J.M. reports funding from the National Institutes of Health, the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality (AHRQ) and US Department of Veterans’ Affairs (VA). B.W.T. reports funding from the VA Office of Rehabilitation Research and Development, the VA Health Services Research and Development Service, and the AHRQ. Her work is supported in part by the Department of Veterans’ Affairs, Veterans’ Health Administration, Office of Research and Development, and the Center for Innovations in Quality, Effectiveness, and Safety (grant no. CIN 13-413). K.C.T. - None.
Conflicts of interest
All authors have no potential conflicts of interest related to this article.