Summary of major changes
This section lists major changes provided in the Strategies to Prevent Catheter-Associated Urinary Tract Infections in Acute-Care Hospitals: 2022 Update,Reference Lo, Nicolle and Coffin1 including recommendations that have been added, removed, or altered. Recommendations in this document are categorized as “essential practices” that are foundational to all HAI programs in acute-care hospitals (in 2014, these were termed “basic practices”) or “additional approaches” to be considered for use in locations and/or populations within hospitals during outbreaks in addition to full implementation of essential practices (in 2014 these were termed “special approaches”). See Table 1 for a complete summary of the recommendations contained in this document.
Essential practices
Updates to the implementation of evidence-based appropriateness criteria for indwelling urethral catheter use
Discussion of strategies for urine-culture stewardship and their impact on CAUTI rates
Updated performance measures to highlight the effects on catheter harm in addition to CAUTI
Discussion of limitations of external urinary catheters
Additional approaches
-
Considerations for injury from urinary catheter use (ie, catheter harm) as well as non–catheter-associated urinary tract infections (eg, UTIs associated with use of alternative urinary collection devices such as external urinary catheters).
-
An updated visual framework for “Disrupting the Life Cycle of Indwelling Urethral Catheter” has been provided to help identify where patient safety interventions can help reduce catheter-associated infection and trauma.
Unresolved issues
-
Standard of care for routine replacement of urinary catheters in place >30 days for the purpose of infection prevention.
-
Best practices for optimizing and tailoring implementation of CAUTI prevention and urine-culture stewardship from the adult acute-care setting to the pediatric acute-care setting.
Intended use
This document was developed following the process outlined in the Handbook for SHEA-Sponsored Guidelines and Expert Guidance Documents.2 No guideline or expert guidance document can anticipate all clinical situations, and this document is not meant to be a substitute for individual clinical judgement by qualified professionals. This document focuses on prevention of catheter-associated urinary tract infections (CAUTIs) in acute-care hospitals. The strategies highlighted may or may not be applicable for other healthcare settings, such as ambulatory settings or long-term or postacute-care facilities. Furthermore, there may be differences in healthcare environments within the hospital (eg, acute-care wards vs intensive care units vs perioperative spaces, etc) that may affect the feasibility of specific recommendations, which should be considered by stakeholders implementing these strategies.
This document is based on a synthesis of evidence, theoretical rationale, current practices, practical considerations, writing group consensus, and consideration of potential harm, where applicable. A summary of the recommendations is provided in Table 1.
Table 1. Summary of Recommendations to Prevent CAUTI

Methods
SHEA recruited 3 subject-matter experts in CAUTI prevention to lead the panel of members representing the Compendium partnering organizations: SHEA, IDSA, APIC, AHA, and The Joint Commission, as well as the Centers for Disease Control and Prevention (CDC).
SHEA utilized a consultant medical librarian who worked with the panel to develop a comprehensive search strategy for PubMed and Embase (January 2012–July 2019; updated to August 2021). Article abstracts were reviewed by panel members in a double-blind fashion using Covidence abstract management software (Covidence, Melbourne, Australia). The articles were subsequently reviewed as full text. The Compendium Lead Authors group voted to update the literature findings, and the librarian reran the search to update it to August 2021. Panel members reviewed the abstracts of these articles via Covidence and incorporated relevant references.
Recommendations resulting from this literature review process were classified based on the quality of evidence and the balance between desirable and potential undesirable effects of various interventions (Table 2). Panel members met via video conference to discuss literature findings, recommendations, quality of evidence for these recommendations, and classification as essential practices, additional approaches, or unresolved issues. Panel members reviewed and approved the document and its recommendations.
Table 2. Quality of Evidencea

a Based on the CDC Healthcare Infection Control Practices Advisory Committee (HICPAC) “Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Recommendations Categorization Scheme for Infection Control and Prevention Guideline Recommendations” (October 2019), the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE)Reference Guyatt, Oxman and Vist179 and the Canadian Task Force on Preventive Health Care.180
The Compendium Expert Panel, made up of members with broad healthcare epidemiology and infection prevention expertise, reviewed the draft manuscript after consensus had been reached by writing panel members.
Following review and approval by the expert panel, the 5 Compendium Partners, stakeholder organizations, and CDC reviewed the document. Prior to dissemination, the guidance document was reviewed and approved by the SHEA Guidelines Committee, the IDSA Standards and Practice Guidelines Committee, AHA, and The Joint Commission, and the Boards of SHEA, IDSA, and APIC. All members complied with SHEA and IDSA policies on conflict-of-interest disclosure.
Section 1: Rationale and statements of concern
Burden of outcomes associated with CAUTI
-
1. Urinary tract infections (UTIs) are one of the most common healthcare-associated infections. In 2003, 70%–80% of UTIs were attributable to the presence of an indwelling urethral catheter. In a 2019 analysis, over 5 years, CAUTIs decreased in proportion to non–device-associated UTIs but still made up an average of 44% of these infections per year among the hospitalized patients included in the study.Reference Saint and Chenoweth3,Reference Strassle, Sickbert-Bennett and Klompas4 The burden of CAUTI in pediatric patients is not well defined; however, bundles adapted from work in adults have been applied to the pediatric population with favorable results.Reference Foster, Ackerman and Hupertz5
-
2. Urinary catheters remain one of the most common medical devices experienced by adults in emergency departments and hospitals worldwide. Often, these devices are placed and maintained in use without an appropriate clinical indication to justify the risk compared to the benefit.Reference Schuur, Chambers and Hou6–Reference Greene, Fakih and Fowler10 Also, 12%–16% of adult hospital inpatients will have an indwelling urethral catheter at some point during admission.Reference Weinstein, Mazon, Pantelick, Reagan-Cirincione, Dembry and Hierholzer11 Of patients who have a urinary catheter placed in the hospital, up to half are placed in patients who may not have an appropriate indication for a urinary catheter.Reference Meddings, Rogers, Krein, Fakih, Olmsted and Saint12
-
3. The daily risk of development of bacteriuria varies from 3% to 7% when an indwelling urethral catheter remains in situ.Reference Garibaldi, Mooney, Epstein and Britt13
-
4. The high frequency of catheter use in hospitalized patients means that the cumulative burden of CAUTI is substantial.Reference Saint and Chenoweth3,Reference Weber, Sickbert-Bennett, Gould, Brown, Huslage and Rutala14–Reference Tambyah, Knasinski and Maki16
-
5. Infection is only one of several adverse outcomes of urinary catheter use. Noninfectious complications include nonbacterial urethral inflammation, urethral strictures, mechanical trauma, and mobility impairment, and these are described in this document as well. The CAUTI rates reported in 2020 for facilities reporting to the National Healthcare Safety Network (NHSN) were 0.754 per 1,000 catheter days for adult inpatient units. At one VA hospital, 0.3% of catheter days involved symptomatic UTI.Reference Leuck, Wright, Ellingson, Kraemer, Kuskowski and Johnson17
-
6. Previous research has shown that CAUTI rates in intensive care units (ICUs) that reported to the NHSN ranged from 1.2 to 4.5 per 1,000 urinary catheter days in adult ICUs and from 1.4 to 3.1 per 1,000 urinary catheter days in pediatric ICUs.Reference Dudeck, Horan and Peterson18 An 8% reduction was observed nationally in CAUTI incidence reported between 2018 and 2019, with the largest decrease noted in ICUs.19,Reference Malpiedi, Peterson and Soe20 The coronavirus disease 2019 (COVID-19) pandemic has affected HAI rates around the world, and data on changes in CAUTI rates were mixed during the pandemic.Reference Weiner-Lastinger, Pattabiraman and Konnor21,Reference Baker, Sands and Huang22
-
7. Bacteremia secondary to CAUTI is infrequent, as demonstrated in a review of 444 episodes of catheter-associated bacteriuria in 308 patients with CAUTI, in which only 3 patients (0.7%) developed bacteremia from a urinary source.Reference Kizilbash, Petersen, Chen, Naik and Trautner23
-
8. CAUTI has been associated with increased mortality and length of stay, but the association with mortality may be a consequence of confounding by unmeasured clinical variables.Reference Chant, Smith, Marshall and Friedrich24 The attributable costs of a CAUTI range from US$603 to US$1,189 for inpatients and up to US$1,764 for patients in ICUs.Reference Hollenbeak and Schilling25,Reference Zimlichman, Henderson and Tamir26
Organizational outcomes associated with CAUTI
Inappropriate use of urine cultures can increase the rates of CAUTI according to the NHSN.Reference Trautner, Grigoryan and Petersen27,Reference Advani, Gao and Datta28 In this document, “NHSN CAUTI” indicates ongoing, thorough review of medical and/or nursing notes in the electronic health record to observe the numbers of urinary catheters in place, urine cultures ordered, and prescribed antimicrobials. Inappropriate treatment of catheter-associated asymptomatic bacteriuria can promote antimicrobial resistance and Clostridioides difficile infection in acute-care facilities. Diagnostic stewardship strategies could be effective in the treatment and prevention of NHSN CAUTI.Reference Trautner, Grigoryan and Petersen27,Reference Luu, Dominguez and Yeshoua29,Reference Al-Bizri, Vahia and Rizvi30
When interventions are implemented to prevent CAUTI, improved outcomes expected are reduced CAUTI, reduced indwelling urethral catheter use, reduced collection and antibiotic use for positive urine cultures, reduced antibiotic-associated complications, and reduced costs associated with these outcomes.
Risk factors for CAUTI
-
1. The duration of catheterization is the most important risk factor for developing infection.Reference Johnson, Roberts, Olsen, Moyer and Stamm31–Reference Riley, Classen, Stevens and Burke33 Accordingly, reducing unnecessary catheter placement and minimizing the duration of catheterization are the primary strategies for CAUTI prevention.
-
2. Additional risk factors include female sex, older age, and not maintaining a closed drainage system.Reference Gould, Umscheid, Agarwal, Kuntz and Pegues34,Reference Hooton, Bradley and Cardenas35 In pediatrics, specific clinical scenarios are often thought to require an open drainage system, including those with recent complex surgical repair or reconstruction of congenital abnormalities of the urogenital system. Available data are insufficient to determine whether specific patients would benefit more from an open drainage system despite an increased risk of CAUTI.Reference Rinke, Oyeku and Heo36,Reference Bryant and Guzman-Cottrill37
-
3. Risk factors for developing healthcare-associated urinary-tract–related bloodstream infection include neutropenia, renal disease, and male sex.Reference Conway, Carter and Larson38–Reference Letica-Kriegel, Salmasian and Vawdrey40
Reservoirs of transmission
-
1. The drainage bag of the bacteriuric patient is a reservoir for organisms that may be transmitted through the hands of healthcare personnel (HCP).Reference Bukhari, Sanderson, Richardson, Kaufman, Aucken and Cookson41
-
2. The drainage bag can also become contaminated by contact with hands due to inadequate hand hygiene, contact with the patient’s skin or hands, or contact with the floor or vessel used to empty the bag.
-
3. Outbreaks of infections associated with resistant gram-negative organisms attributable to bacteriuria in catheterized patients have been reported.Reference Schaberg, Weinstein and Stamm42–Reference Yoon, Choi and Park44
Section 2: Background on definitions of CAUTI
The clinical diagnosis of CAUTI is often a diagnosis of exclusion,Reference Hooton, Bradley and Cardenas35 making it difficult to have a standardized definition. At present, all of the available definitions have substantial limitations.Reference Advani, Fakih, Weber and Talbot45 We discuss the criteria, advantages, and limitations of different definitions used for CAUTI in Table 4.
Table 3. Process and Outcome Measures for CAUTI and Other Catheter Harms
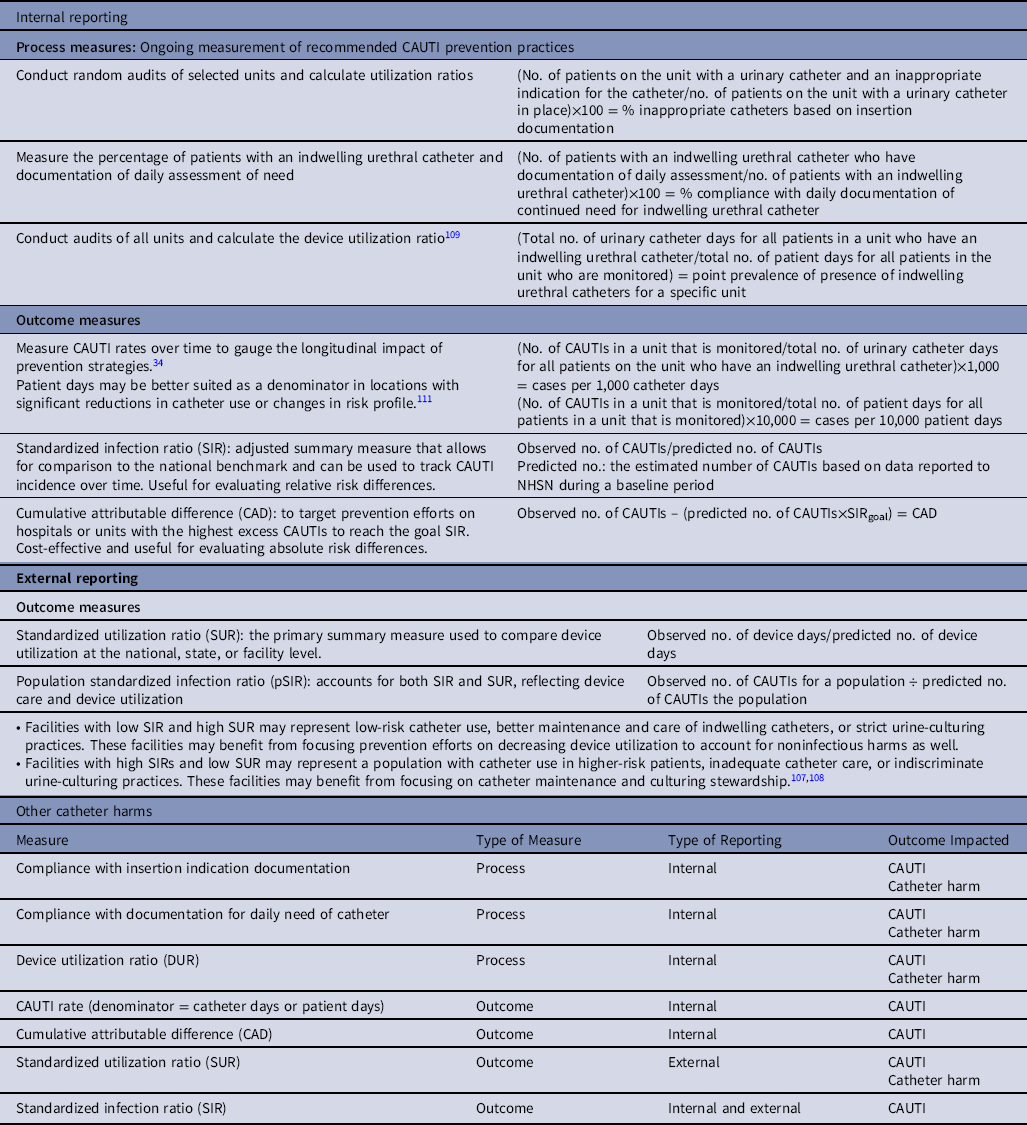
Table 4. Criteria, Advantages, and Limitations of Definitions Used for Identifying CAUTIsa

a Adapted from Advani SD, Fakih MG. Health Care–Associated Urinary Tract Infections (Including CAUTI), Mayhall’s Hospital Epidemiology and Infection PreventionReference Advani, Fakih, Weber and Talbot45
The optimal definition for CAUTI used for surveillance and quality improvement is one that only captures true instances of symptomatic infection that would benefit from antimicrobial treatment. The NHSN CAUTI definition has been adopted nationally, but other definitions are also used for clinical care and administrative purposes.46 The NHSN CAUTI definition has been updated in 2015 with exclusion of yeast as a pathogen and increase in the urine-culture bacterial threshold to ≥105 colony-forming units per milliliter (CFU/mL) to align with a clinical definition for symptomatic CAUTI.Reference Bardossy, Jayaprakash and Alangaden47,Reference Advani, Lee, Schmitz and Camins48
Section 3: Background on prevention of CAUTI
Summary of existing guidelines and recommendations
(See Supplementary Content, Appendix 1 online)
-
1. The CDC published guidelines for prevention of CAUTI in 1981, and these were updated in 2009.Reference Gould, Umscheid, Agarwal, Kuntz and Pegues34 These guidelines provide recommendations for catheter use, catheter insertion, catheter care, and implementation of programs to prevent CAUTI.
-
2. The CDC also developed the Targeted Assessment for Prevention (TAP) Strategy as a framework for quality improvement that uses data for action to prevent HAIs,49 including CAUTIs. The following 3 components of the TAP Strategy focus on CAUTI:
-
a. Running TAP reports in the NHSN to target healthcare facilities and specific units with excess CAUTIs.
-
b. Applying TAP Facility Assessment Tools to identify gaps in infection prevention in the targeted locations.
-
c. Accessing infection prevention resources within the TAP CAUTI Implementation Guide49 to address identified gaps in CAUTI prevention.
-
-
3. The IDSA together with other professional societies published international guidelines for the management of CAUTI in 2010.Reference Hooton, Cardenas and Bradley50
-
4. The Department of Health in Great Britain published guidelines for preventing infections associated with the insertion and maintenance of short-term indwelling urethral catheters in acute care in 2001,Reference Panknin and Althaus51 updated in 2014.Reference Pratt, Pellowe and Wilson52
-
5. Pragmatic tools for reducing inappropriate use of indwelling urethral catheters and antibiotics have also been published by organizations of hospitalists,Reference Trautner, Prasad and Grigoryan53 nurses, and other funders of interventions to improve safety are also readily accessible for use, including resources targeting acute-care, long-term care, and ambulatory settings.
Conceptual models and frameworks for prioritizing interventions to prevent CAUTI
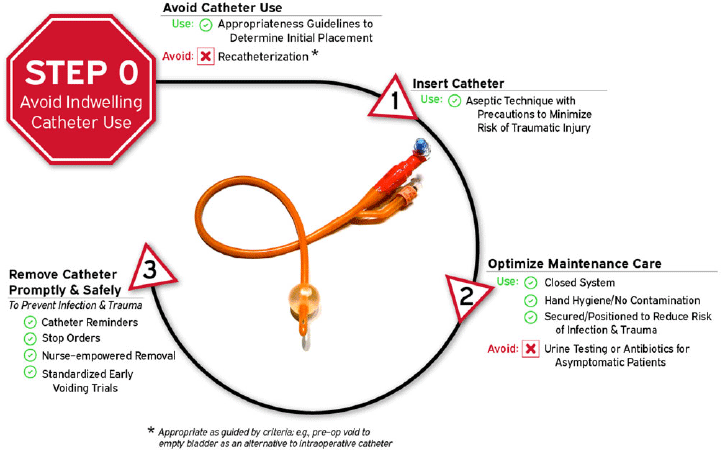
Given the number of intervention opportunities for reducing urinary-catheter–associated complications, a conceptual model known as “Disrupting the Life Cycle of the Urinary Catheter” may help to assess the comprehensiveness of a hospital’s or unit’s strategies for preventing catheter-associated complications, including CAUTI.Reference Meddings and Saint54 This conceptual model has been used in recent large-scale collaboratives funded by the CDC and the Agency for Healthcare Research and Quality (AHRQ) in teaching materials to summarize critical types of interventions to consider in a comprehensive CAUTI prevention program.Reference Patel, Gupta, Vaughn, Mann, Ameling and Meddings55–Reference Meddings, Manojlovich and Ameling57 As illustrated in Figure 1 and adapted for this current document, the most important step (Life Cycle Step 0, labeled as Step 0 because it occurs before a urinary catheter’s “life” (or existence) as a medical device placed in the patient) to prevent both infectious and noninfectious catheter complications is avoiding placement of the indwelling catheter whenever possible. Life Cycle Step 0 includes employing non-catheter urinary management strategies such as prompted toileting, urinals, bedside commodes, and incontinence garments, and/or non–indwelling-catheter strategies such as intermittent straight catheterization (ISC) or consideration of external urinary catheters (EUCs).Reference Zavodnick, Harley, Zabriskie and Brahmbhatt58,Reference Eckert, Mattia, Patel, Okumura, Reynolds and Stuiver59 Literature for EUCs related to cost-effectiveness, risks of EUC–associated skin injury or UTIs compared to other strategies is still evolving.Reference Zavodnick, Harley, Zabriskie and Brahmbhatt58–Reference Warren, Fosnacht and Tremblay62 Refer to Table 5 for summary of recent literature on CAUTI prevention initiatives.
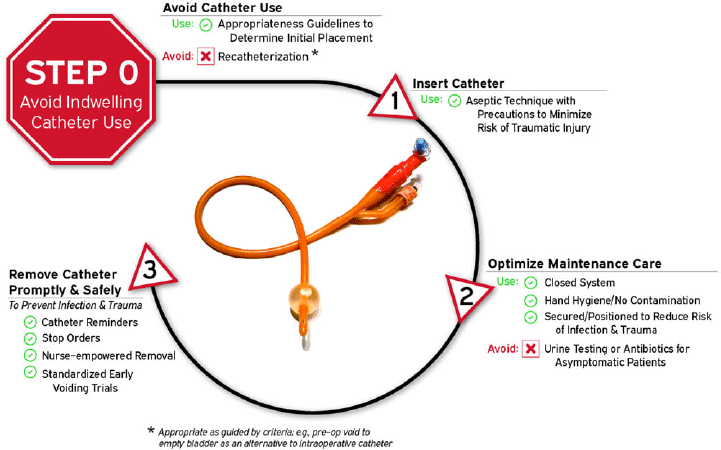
Figure 1. Disrupting the life cycle of the indwelling urethral catheter to reduce catheter-associated infection and trauma.
Table 5. CAUTI Literature

Section 4: Recommended strategies for CAUTI prevention
Recommendations are categorized either as essential practices that should be adopted by all acute-care hospitals or as additional approaches that can be considered for use in locations and/or populations within hospitals when CAUTIs are not controlled using essential practices. Essential practices include recommendations in which the potential to impact CAUTI risk clearly outweighs the potential for undesirable effects. Additional approaches include recommendations in which the intervention is likely to reduce CAUTI risk but there is concern about the risks for undesirable outcomes, the quality of evidence is low, or evidence supports the impact of the intervention in select settings (eg, during outbreaks) or for select patient populations. Hospitals can prioritize their efforts by initially focusing on implementing essential practices. If CAUTI surveillance or other risk assessments suggest that ongoing opportunities for improvement exist, hospitals should then consider adopting some or all of the additional approaches. These interventions can be implemented in specific locations or patient populations or can be implemented hospital-wide, depending on outcome data, risk assessment, and/or local requirements. Each infection prevention recommendation is evaluated for quality of evidence (Table 2). Recommendations for preventing and monitoring CAUTIReference Gould, Umscheid, Agarwal, Kuntz and Pegues34,Reference Hooton, Bradley and Cardenas35,Reference Panknin and Althaus51,Reference Pratt, Pellowe and Wilson52 are summarized in the following section and Table 1.
Essential practices for preventing CAUTI: Recommended for all acute-care hospitals
-
1. Perform a CAUTI risk assessment and implement an organization-wide program to identify and remove catheters that are no longer necessary using 1 or more methods documented to be effective.Reference Gould, Umscheid, Agarwal, Kuntz and Pegues34,Reference Hooton, Bradley and Cardenas35,Reference Panknin and Althaus51,Reference Pratt, Pellowe and Wilson52 (Quality of evidence: MODERATE)
-
a. Develop and implement institutional policy requiring periodic, usually daily, review of the necessity of continued catheterization.
-
b. Consider utilizing electronic or other types of reminders (see Supplementary Content, Appendices 2 and 3 online) of the presence of a catheter and required criteria for continued use.Reference Stark and Maki63 Examples include the following:
-
i. Automatic stop orders requiring review of current indications and renewal of order for continuation of the indwelling catheter.
-
ii. Standardized reminders of persistent catheters together with current catheter indications (see Supplementary Content, Appendices 2 and 3 online) targeting either physicians or nurses.
-
-
c. Nursing and physician staff conduct daily reviews during rounds of all patients with urinary catheters to ascertain necessity of continuing catheter use.Reference Siegel, Figueroa and Stockwell64
-
-
2. Provide appropriate infrastructure for preventing CAUTI.Reference Meddings, Greene and Ratz56 (Quality of evidence: LOW)
-
a. Ensure that the supplies for following best practices for managing urinary issues are readily available to staff in each unit, including bladder scanners, non–catheter-incontinence management supplies (urinals, garments, bed pads, skin products), male and female external urinary catheters, straight urinary catheters, and indwelling catheters including the option of catheters with coude tips.
-
b. Ensure that non-catheter urinary management supplies are as easy to obtain for bedside use as indwelling urinary catheters.
-
c. Ensure the physical capability for urinary catheters with tubes attached to patients (eg, indwelling urinary catheters and some external urinary catheters [EUCs]) to be positioned on beds, wheelchairs, at an appropriate height and without kinking for patients in their rooms and during transport.
-
-
3. Provide and implement evidence-based protocols to address multiple steps of the urinary catheter life cycle (Fig. 1): catheter appropriateness (step 0), insertion technique (step 1), maintenance care (step 2), and prompt removal (step 3) when no longer appropriate. (Quality of evidence: LOW)
-
a. Adapt and implement evidence-based criteria for acceptable indications for indwelling urethral catheter use, which may be embedded as standardized clinical-decision support tools within electronic medical record (EMR) ordering systems. Expert-consensus–derived indications for indwelling catheter use have been developed, although research that assesses the appropriateness of these uses is limited.Reference Gould, Umscheid, Agarwal, Kuntz and Pegues34,Reference Meddings, Saint and Fowler65 The limited examples of appropriate indications for indwelling urethral catheter include the following:
-
i. Perioperative use for selected surgical proceduresReference Meddings, Skolarus and Fowler66,Reference Skolarus, Dauw, Fowler, Mann, Bernstein and Meddings67 such as urologic surgery or surgery on contiguous structures of the genitourinary tract, prolonged surgery, large-volume infusions or diuretics during surgery, or intraoperative monitoring of urine output is needed. Notably, if a catheter is placed intraoperatively simply due to the duration of surgery (eg, >3 hours) or for decompression to address a specific surgical approach, then such catheters should be removed at the end of the surgical case.
-
ii. Hourly assessment of urine output in ICU patients when used clinically to modify therapies frequently such as volume resuscitation, diuresis, and vasopressors. ICU care alone is not an appropriate justification for indwelling catheter placement; a specific clinical indication is still needed.
-
iii. Management of acute urinary retentionReference Meddings, Saint and Fowler65,68 (eg, new retention of urine with postvoid residual bladder volume >500 cm3 by bladder scanner if no symptoms, or >300 cm3 if having symptoms such as bladder pain or fullness, persistent urge to void, new incontinence or leaking, or only able to have frequent small voids)
-
iv. Assistance in healing of open pressure ulcers or skin grafts for selected patients with urinary incontinence when alternative supplies for protective wound or managing incontinence (eg, external urinary catheters, wound dressings) are not feasible.
-
v. Facilities may allow exceptions as part of a palliative and/or comfort care regimen, if use of the catheter addresses a specific goal of the patient, such as reducing the need for frequent bed or garment changes or preventing pain that cannot be well managed.
-
-
-
4. Ensure that only trained HCP insert urinary catheters and that competency is assessed regularly.Reference Meddings, Saint and Fowler65 (Quality of evidence: LOW)
-
a. Require supervision by experienced HCP when trainees insert and remove catheters to reduce the risk of infectious and traumatic complications related to urinary catheter placement.Reference Leslie, Sajjad and Sharma69–Reference Barnum, Tatebe, Halverson, Helenowski, Yang and Odell71 Given much higher rates of CAUTI when catheters are placed by trainees such as medical students,Reference Barnum, Tatebe, Halverson, Helenowski, Yang and Odell71,Reference Sultan, Kilic, Arnaoutakis and Kilic72 educational programs may need to reassess at which point in medical training and which trainees specifically are most appropriate for being involved in urinary catheter insertion in patients compared to simulation models only.
-
-
5. Ensure that supplies necessary for aseptic technique for catheter insertion are available and conveniently located. (Quality of evidence: LOW)
-
6. Implement a system for documenting the following in the patient record: physician order for catheter placement, indications for catheter insertion, date and time of catheter insertion, name of individual who inserted catheter, nursing documentation of placement, daily presence of a catheter and maintenance care tasks, and date and time of catheter removal. Record criteria for removal and justification for continued use. (Quality of evidence: LOW)
-
a. Record in a standard format for data collection and quality improvement purposes and keep accessible documentation of catheter placement (including indication) and removal.
-
b. If available, utilize electronic documentation that is searchable.
-
c. Consider nurse-driven urinary catheter removal protocols for first trial of void without an indwelling catheter when the indication for placement has resolved (see Essential Practices, 3). These protocols can be implemented as part of the routine urinary catheter placement order or as an expected reminder (or “standing order”) from the nurse to the physician in rounds. These protocols should list some exceptions or “opt outs,” such as for postoperative urology surgery patients or patients for whom a catheter required urology consult for placement that should not be removed without physician order.
-
-
7. Ensure that sufficiently trained HCP and technology resources are available to support surveillance for catheter use and outcomes.Reference Hsu, Shenoy and Kelbaugh73 (Quality of evidence: LOW)
-
8. Perform surveillance for CAUTI if indicated based on facility risk assessment or regulatory requirements as described in Section 5.Reference Hsu, Shenoy and Kelbaugh 73 (Quality of evidence: LOW)
-
9. Standardize urine culturing by adapting an institutional protocol for appropriate indications for urine cultures in patients with and without an indwelling catheter.Reference Trautner, Grigoryan and Petersen27,Reference Trautner, Prasad and Grigoryan53,Reference Mullin, Kovacs and Fatica74–Reference Munigala, Jackups and Poirier76 Consider incorporating these indications into the EMR, and review indications for ordering urine cultures in CAUTI risk assessment.Reference Claeys, Trautner and Leekha77 (Quality of evidence: LOW)
Education and training
-
1. Educate HCP involved in the insertion, care, and maintenance of urinary catheters about CAUTI prevention, including alternatives to indwelling catheters, and procedures for catheter insertion, management, and removal.Reference Blondal, Ingadottir and Einarsdottir78 (Quality of evidence: LOW)
-
2. Assess healthcare professional competency in catheter use, catheter care, and maintenance.Reference Mody, Saint, Galecki, Chen and Krein79–Reference Huang, Hong, Zhao, Lin, Xi and Knowledge81 (Quality of evidence: LOW)
-
3. Educate HCP about the importance of urine culture stewardship and provide indications for urine cultures. (Quality of evidence: LOW)
-
a. Consider requiring clinicians to identify an appropriate indication for urine culturing when placing an order for a urine culture. The indication should be supported by the literatureReference Luu, Dominguez and Yeshoua29,82–Reference Morgan, Croft and Deloney86 and should be appropriate for the specific patient population. Include guideline-based remindersReference Nicolle, Gupta and Bradley87 for the specific circumstances. Below is a simple example of appropriate and inappropriate reasons to culture urine are referenced online on the CDC website,82,83 though several other lists are available in the literature specific to particular clinical settings (eg, ICU, emergency department, nursing home, and catheterized versus non-catheterized patients):
-
i. Appropriate uses of urine culture include the following:
-
a. Presence of symptoms suggestive of a urinary tract infection (UTI):
i. Flank pain or costovertebral angle tenderness
ii. Acute hematuria
iii. New pelvic discomfort
-
b. New onset or worsening sepsis without evidence of another source on history, physical examination, or laboratory testing
-
c. Fever or altered mental status without evidence of another source on history, physical examination, or laboratory testing
-
d. In spinal-cord-injury patients and other highly complex patients (eg, patients with >40% total body burn, recipients of kidney transplants with graft failure) symptoms may include increased spasticity, autonomic dysreflexia, and/or sense of unease.
-
-
ii. Inappropriate uses of urine cultures include the following:
-
a. Odorous, cloudy, or discolored urine in the absence of other localizing signs and symptoms
-
b. Reflex urine cultures based on urinalysis results, such as pyuria, in the absence of other indications (absence of pyuria suggests diagnosis other than CAUTI)
-
c. Urine culture to document response to therapy unless symptoms fail to resolve.
-
-
-
-
4. Provide training on appropriate collection of urine. Specimens should be collected and should arrive at the microbiology laboratory as soon as possible, preferably within an hour. If delay in transport to the laboratory is expected, samples should be refrigerated (no more than 24 hours) or collected in preservative urine transport tubes. (Quality of evidence: LOW)
-
5. Train clinicians to consider other methods for bladder management (eg, intermittent catheterization, or external male or female collection devices) when appropriate before placing an indwelling urethral catheter. (Quality of evidence: LOW)
-
6. Share data in a timely fashion and report to appropriate stakeholders. (Quality of evidence: LOW)
Insertion of indwelling catheters
-
1. Insert urinary catheters only when necessary for patient care and leave in place only as long as indications remain. (Quality of evidence: MODERATE)
-
2. Consider other methods for bladder management such as intermittent catheterization, or external male or female collection devices, when appropriate. (Quality of evidence: LOW)
-
3. Use appropriate technique for catheter insertion. (Quality of evidence: MODERATE)
-
4. Consider working in pairs to help perform patient positioning and monitor for potential contamination during placement.Reference Fletcher-Gutowski and Cecil88–Reference Barry, Allen, Chlebeck, Siebenaler, Wick and Gunderson90 (Quality of evidence: LOW)
-
5. Practice hand hygiene (based on CDC or World Health Organization guidelines) immediately before insertion of the catheter and before and after any manipulation of the catheter site or apparatus. (Quality of evidence: LOW)
-
6. Insert catheters following aseptic technique and using sterile equipment. (Quality of evidence: LOW)
-
7. Use sterile gloves, drape, and sponges, a sterile antiseptic solution for cleaning the urethral meatus, and a sterile single-use packet of lubricant jelly for insertion. (Quality of evidence: LOW)
-
8. Use a catheter with the smallest feasible diameter consistent with proper drainage to minimize urethral trauma but consider other catheter types and sizes when warranted for patients with anticipated difficult catheterization to reduce the likelihood that a patient will experience multiple, sometimes traumatic, catheterization attempts. (Quality of evidence: LOW)
Management of indwelling catheters
-
1. Properly secure indwelling catheters after insertion to prevent movement and urethral traction.Reference Willson, Wilde and Webb91,92 (Quality of evidence: LOW)
-
2. Maintain a sterile, continuously closed drainage system.92,Reference Manojlovich93 (Quality of evidence: LOW)
-
3. Replace the catheter and the collection system using aseptic technique when breaks in aseptic technique, disconnection, or leakage occur. (Quality of evidence: LOW)
-
4. For examination of fresh urine, collect a small sample by aspirating urine from the needleless sampling port with a sterile syringe or cannula adaptor after cleansing the port with disinfectant. (Quality of evidence: LOW)
-
5. Facilitate timely transport of urine samples to laboratory. If timely transport is not feasible, consider refrigerating urine samples or using sample collection cups with preservatives. Obtain larger volumes of urine for special analyses (eg, 24-hour urine) aseptically from the drainage bag. (Quality of evidence: LOW)
-
6. Maintain unobstructed urine flow. (Quality of evidence: LOW)
-
a. Remind bedside caregivers, patients, and transport personnel to always keep the collecting bag below the level of the bladder.
-
b. Do not place the bag on floor.
-
c. Keep the catheter and collecting tube free from kinking, which can impair urinary flow and increase stasis within the bladder, increasing infection risk.
-
d. Empty the collecting bag regularly using a separate collecting container for each patient. Avoid touching the draining spigot to the collecting container.
-
-
7. Employ routine hygiene. Cleaning the meatal area with antiseptic solutions is an unresolved issue, though emerging literature supports chlorhexidine use prior to catheter insertion.Reference Mitchell, Curryer, Holliday, Rickard and Fasugba94–Reference Fasugba, Koerner, Mitchell and Gardner97 Alcohol-based products should be avoided given concerns about the alcohol causing drying of the mucosal tissues (Photos of these steps are available).92,Reference Manojlovich93 (Quality of evidence: LOW)
Additional approaches for preventing CAUTI
These additional approaches are recommended for use in locations and/or populations within the hospital with unacceptably high CAUTI rates or standardized infection ratios (SIRs) despite implementation of the essential CAUTI prevention strategies listed previously.
-
1. Develop a protocol for standardizing diagnosis and management of postoperative urinary retention, including nurse-directed use of intermittent catheterization and use of bladder scannersReference Brackmann, Carballo, Uppal, Torski, Reynolds and McLean98,Reference Bjerregaard, Homilius, Bagi, Hansen and Kehlet99 when appropriate as alternatives to indwelling urethral catheterization. (Quality of evidence: MODERATE)
-
a. If bladder scanners are used, clearly state indications, train nursing staff in their use, and disinfect between patients according to manufacturer’s instructions.
-
-
2. Establish a system for analyzing and reporting data on catheter use and adverse events from catheter use. (Quality of evidence: LOW)
-
a. Use cumulative attributable difference to identify high-risk units or hospitals (as described in Section 5).
-
b. Measure process and outcomes measures (eg, standardized utilization ratio and standardized infection ratio) as described in Section 5.
-
c. Define and monitor catheter harm (see Fig. 2) in addition to CAUTI, including catheter obstruction, unintended removal, catheter trauma, or reinsertion within 24 hours of removal.Reference Saint, Trautner and Fowler100
-
i. National focus has been on CAUTI prevention, but current metrics do not adequately capture overall catheter harm.Reference Fakih and Advani101 Catheter harm includes infectious complications in addition to CAUTI (eg, secondary bacteremia, asymptomatic bacteriuria consequences) and noninfectious catheter complications (Fig. 2).
-
ii. Patients with an indwelling urethral catheter are 5 times more likely to experience noninfectious complications (eg, urethral injury, pain, or inadvertent catheter removal) than infectious complications.Reference Saint, Trautner and Fowler100
-
iii. Current metrics used to monitor progress in the prevention of CAUTIs may underestimate both infectious and noninfectious catheter harm.
-
-
-
3. Establish a system for defining, analyzing, and reporting data on non–catheter-associated UTIs, particularly UTIs associated with devices used as alternatives to indwelling urethral catheters. (Quality of evidence: LOW)
-
a. Non–catheter-associated UTIs are defined as UTIs that occur in hospitalized patients without an indwelling urethral catheter. These include but are not limited to patients who have had no urinary device at all, as well as those with external urinary catheters (EUCs), urinary stents, or urostomies, or who undergo intermittent catheterization, and thus are not captured by the NHSN CAUTI definition.
-
b. As the incidence of CAUTI continues to decline, the proportion of non–catheter-associated UTIs is increasing in some hospitals.Reference Strassle, Sickbert-Bennett and Klompas4 However, the national incidence of non–catheter-associated UTIs is not known because surveillance and reporting of these UTIs are not required by US Federal agencies.
-
c. Non–catheter-associated UTIs are a common indication for antibiotics in hospitalized patients, and this metric could provide important information as healthcare facilities consider the risks and benefits of newer alternatives to urinary catheters with currently limited published data on adverse events (eg, EUCs for women) to help inform when the benefit outweighs the potential risk for specific patient populations.
-
Approaches that should not be considered a routine part of CAUTI prevention
-
1. Routine use of antimicrobial- or antiseptic-impregnated catheters. (Quality of evidence: HIGH)
-
2. Breaking a closed system. (Quality of evidence: LOW)
-
3. Screening for asymptomatic bacteriuria in catheterized patients, except in the few patient populations for which this is anticipated to have more benefit than harm, as detailed in the 2019 IDSA Guideline for Management of Asymptomatic Bacteriuria102 and the 2019 US Preventative Services Task Force Recommendation on Asymptomatic Bacteriuria in Adults102 (eg, pregnant women, patients undergoing endoscopic urologic procedures associated with mucosal trauma). (Quality of evidence: HIGH)
-
a. Treatment of asymptomatic bacteriuria is not an effective strategy to prevent CAUTI in other patient groups, as it increases the risk of antibiotic-associated complications more than any potential benefit for the prevention of symptomatic CAUTI. The conditions that predispose the patient to bladder colonization (anatomic, immunologic) are not resolved by antibiotics, so bacteriuria recurs.
-
-
4. Catheter irrigation as a strategy to prevent infection. (Quality of evidence: MODERATE)
-
a. Do not perform continuous irrigation of the bladder with antimicrobials as a routine infection prevention measure.
-
b. If continuous irrigation is being used to prevent obstruction, maintain a closed system.
-
-
5. Routine use of systemic antimicrobials as prophylaxis. (Quality of evidence: LOW)
-
6. Routine changing of catheters to avoid infection. (Quality of evidence: LOW)
-
a. In the case of a patient with a long-term catheter in place (ie, >7 days), catheter replacement can be considered at the time of specimen collection for urine testing to obtain a fresh sample.Reference Frontera, Wang and Phillips103,104
-
-
7. Alcohol-based products on the genital mucosa. (Quality of evidence: LOW)
Unresolved issues and future areas of study
-
1. Use of antiseptic solution versus sterile saline for meatal and perineal cleaning prior to catheter insertion.Reference Mitchell, Curryer, Holliday, Rickard and Fasugba94–Reference Mitchell, Fasugba and Cheng96,Reference Clark and Wright105
-
2. Use of urinary antiseptics (eg, methenamine) to prevent UTI.
-
3. Spatial separation of patients with urinary catheters in place to prevent transmission of pathogens that could colonize urinary drainage systems.
-
4. Standard of care for routine replacement of urinary catheters in place >30 days for infection prevention.Reference Cooper, Alexander, Sinha and Omar106
-
5. Best practices for optimizing and tailoring implementation of CAUTI prevention and urine-culture stewardship from the adult acute-care setting to the pediatric acute-care setting.
Section 5: Performance measures
Different performance measures have been used for internal and external reporting of CAUTIs and catheter utilization. Hospitals may use a combination of metrics for public reporting and to assess the impact of quality improvement initiatives. Here, we discuss outcome and process measures for both internal and external reporting.
Internal reporting
These performance measures are intended to support internal hospital quality improvement efforts and do not necessarily address external reporting requirements. The process and outcome measures suggested here are derived from published guidelines, other relevant literature, and the opinions are those of the authors. These process and outcome measures can be shared with senior hospital leadership, nursing leadership, and clinicians who care for patients at risk for CAUTI.Reference Advani, Smith, Seidelman, Turner, Anderson and Lewis107,Reference Pepe, Maloney and Leung108
Process measures
-
1. Percentage of inappropriate catheters based on insertion documentation
-
a. Conduct random audits of selected units and calculate utilization ratios:
-
i. Numerator: number of patients on the unit with a urinary catheter and an inappropriate indication for the catheter.
-
ii. Denominator: number of patients on the unit with a urinary catheter in place.
-
iii. Multiply by 100 so that the measure is expressed as a percentage.
-
-
-
2. Percent compliance with daily documentation of continued need for indwelling urethral catheter
-
a. Measure the percentage of patients with an indwelling catheter and documentation of daily assessment of need
-
i. Numerator: number of patients with an indwelling catheter who have documentation of daily assessment.
-
ii. Denominator: number of patients with an indwelling catheter.
-
iii. Multiply by 100 so that the measure is expressed as a percentage.
-
-
-
3. Point prevalence of indwelling urethral catheters for a specific unit
-
a. Conduct audits of all units and calculate the device utilization ratioReference Fakih, Gould and Trautner109
-
i. Numerator: total number of urinary catheter days for all patients in a unit who have an indwelling urethral catheter.
-
ii. Denominator: total number of patient days for all patients in a unit who are monitored.
-
iii. Divide the numerator by the denominator to get point prevalence of presence of indwelling urethral catheters (for a specific unit).
-
-
Outcome measures
-
1. CAUTI rates, stratified by risk factors (eg, ward, clinical service line)
-
a. Measure CAUTI rates over time to gauge the longitudinal impact of prevention strategiesReference Gould, Umscheid, Agarwal, Kuntz and Pegues34
-
i. Numerator: number of CAUTIs in a unit that is monitored.
-
ii. Denominators
-
1. Total number of urinary catheter days for all patients on the unit who have an indwelling urethral catheter.
-
2. Total number of patient days for all patients in a unit that is monitored.
-
-
iii. Multiply by 1,000 so that the measure is expressed as cases per 1,000 catheter days, or 10,000 to express as cases per 10,000 patient days.Reference Horstman, Li, Almenoff, Freyberg and Trautner110 Patient days may be better suited as the denominator in locations with significant reductions in catheter use or changes in risk profile (eg, removing low-risk catheters in ED or ICU leaves a population of high-risk catheters).Reference Wright, Kharasch, Beaumont, Peterson and Robicsek111
-
-
-
2. Standardized infection ratio (SIR)
-
a. The SIR is a risk-adjusted summary measure that allows for a comparison to the national benchmark and can be used to track CAUTI incidence over time.
-
b. The ratio is calculated by dividing the observed number of CAUTIs by the predicted number of CAUTIs.
-
c. The predicted number of infections is an estimated number of CAUTIs based on infections reported to NHSN during a baseline period (currently 2015 for CAUTI, risk-adjusted for patient care location and facility characteristics).
-
-
3. Cumulative attributable difference (CAD)
-
a. The cumulative attributable difference (CAD) is used in the CDC Targeted Assessment for Prevention (TAP) Strategy to target prevention efforts to hospitals or units with the highest excess HAIs.Reference Soe, Gould, Pollock and Edwards112 The CAD is the number of excess infections that need to be prevented to reach a goal SIR (set by the end user) as shown below:
-
i. CAD = observed number of CAUTIs − prevention target (predicted×SIRgoal)
-
ii. CAD is a cost-effective strategy used for targeting and prioritizing units.
-
-
The SIR is useful for evaluating relative risk differences, while the CAD is useful for evaluating absolute risk differences.
Internal reporting can be strengthened in the future by developing and refining metrics that target rate of urine culturing as well as compliance with urine collection techniques in patients with and without indwelling catheters.
External reporting
There are many challenges in providing useful information to consumers and other stakeholders in public reporting of HAIs.Reference Wong, Rupp and Mermel113 Recommendations for public reporting of HAIs have been provided by the Healthcare Infection Control Practices Advisory Committee (HICPAC),Reference McKibben, Horan and Tokars114 the Healthcare-Associated Infection Working Group of the Joint Public Policy Committee, and the National Quality Forum (NQF). In January 2012, most acute-care facilities began reporting CAUTIs from adult and pediatric ICUs to the NHSN to meet requirements of the Centers for Medicare and Medicaid Services (CMS) Inpatient Prospective Payment System FY2012 final rule.
The validity of the current CDC NHSN definition for CAUTI for comparing facility-to-facility outcomes has not been established, so caution is recommended when performing interfacility comparisons of CAUTI rates. Use of hospital claims data to compare healthcare-associated CAUTI rates also has not been validated.Reference Meddings and McMahon115 Choice of metrics may also be influenced by hospital size. For example, SIR may be more suitable for larger hospitals or hospitals with higher CAUTI events, while SUR or “days since last CAUTI” may be a more useful metric for smaller hospitals or hospitals with rare events.Reference Advani, Smith, Seidelman, Turner, Anderson and Lewis107,Reference Pepe, Maloney and Leung108
State and local requirements
Hospitals in states that have mandatory reporting requirements must collect and report the data required by the state. For information on state and federal requirements, check with your state or local health department.
External quality initiatives
Hospitals that participate in external quality initiatives must collect and report the data required by the initiative.
Outcome measures
-
1. Standardized utilization ratio (SUR): The SUR is the primary summary measure used to compare device utilization at the national, state, or facility level.
-
a. This ratio is calculated by dividing the observed number of device days by the predicted number of device days.
-
b. The predicted number of device days is an estimated number of device days based on data reported to NHSN during a baseline period (currently 2015 for CAUTI, risk-adjusted for patient care location and facility characteristics).
-
-
2. Standardized infection ratio (SIR): The SIR is a summary measure used to track HAIs at a national, state, or facility level over time, and has been described above.
-
a. Consider measures that address device risk at the patient population level. A population SIR (pSIR) accounts for both SIR and SUR, reflecting both device care and device utilization.
-
Using different metrics in combination is a better strategy than using a single metric to target prevention efforts. For example, hospitals with low SIRs and high device utilization may represent low-risk catheter use, better maintenance and care of indwelling catheters, or strict urine-culturing practices. In these scenarios, focusing prevention efforts on decreasing device utilization should be considered to account for noninfectious catheter harm as well. Alternatively, hospitals that have high SIRs and low device utilization may represent a population with more high-risk catheter use (ie, catheters in high-risk patients), inadequate catheter care, or indiscriminate urine culturing practices. These hospitals may benefit from focusing on catheter maintenance and stewardship of culturing.Reference Advani, Smith, Seidelman, Turner, Anderson and Lewis107,Reference Pepe, Maloney and Leung108
Section 6: Implementation strategies
Preventing CAUTI requires a focus on both technical and socioadaptive (or behavioral) components.Reference Saint, Meddings and Chopra116 Although the general concepts of implementation science are found in a companion article of the 2022 Compendium, we detail here some of the CAUTI-specific implementation work—and lessons learned—those hospitals should be aware of since the 2014 update.Reference Yokoe, Anderson and Berenholtz117 Interventions to assist with program implementation and evaluation that have been reported to be associated with improved outcomes are provided in this section. These interventions are often multifaceted and generally include elements of the “4E’s” model.Reference Pronovost, Berenholtz and Needham118 This section is particularly aimed toward physicians, nurses, and infection preventionists that are in leadership positions, to review when designing or revising strategies to optimize implementation of CAUTI prevention strategies.
Over the past several years, regional and national CAUTI prevention initiatives have been implemented in acute-care hospitals. Several of these initiatives have been successfulReference Saint, Greene, Kowalski, Watson, Hofer and Krein119–Reference Saint, Greene and Krein121 but not in all patient care locations or settings.Reference Meddings, Greene and Ratz56,Reference Meddings, Manojlovich and Ameling122 In 2013, investigators in Michigan published the results of a comparison study in which they surveyed infection preventionists nationally (with an oversample of Michigan hospitals) and also evaluated SIRs comparing Michigan with non-Michigan hospitals.Reference Saint, Greene, Kowalski, Watson, Hofer and Krein119 In this study, hospitals that were more likely to participate in collaboratives aimed at reducing HAIs, more likely to use bladder scanners, stop orders, reminders, or nurse-initiated removal of urinary catheters, corresponded to a 25% reduction of CAUTI rates compared with other hospitals.Reference Saint, Greene, Kowalski, Watson, Hofer and Krein119
In a study of 7 Veterans’ Affairs (VA) hospitals, a multidisciplinary team demonstrated a significant reduction in CAUTI rates on medical-surgical wards (from 2.4 to 0.8 CAUTI per 1,000 catheter days; P = .001) but no significant change in ICU CAUTI rates.Reference Saint, Fowler and Sermak120 The intervention used a 2-tier system of interventions to determine each hospital’s specific needs relative to their CAUTI rates. Tier 1 describes data-gathering needs and a nursing template in the EMR to prompt staff to consider removing catheters at the earliest possible point. This tier alone can create sufficient visibility within the organization to reduce CAUTI rates to the desired level. Tier 2 is a more intensive tier of steps that hospitals can implement for stubborn rates that will not come down, including root-cause analysis. In a separate study by Saint et al, the team implemented a multifactorial intervention at >900 non-VA hospital units in the United States. This intervention utilized the Comprehensive Unit-Based Safety Program (CUSP). As with the Saint VA hospital study, this intervention led to a reduction in CAUTI rates in non-VA hospital medical-surgical wards (from 2.40 to 2.05 CAUTIs per 1,000 catheter days) but not in ICUs.Reference Saint, Greene and Krein121 These 2 CAUTI prevention efforts in VA and non-VA hospitals have thus far failed to significantly reduce rates in ICUs, likely related to several reasons including cultural differences between critical-care units and medical-surgical floors in the United States. Specifically, critical-care units often consider indwelling urethral catheters to be a standard of care for their patients,Reference Huang123 even when suitable alternatives could be used.Reference Meddings, Saint and Fowler65,Reference Huang123 The CUSP model was also used to focus entirely on ICUs with elevated baseline rates of CAUTI; this negative study revealed a need to develop a new model for helping such struggling hospitals. In the nursing home setting, as part of the AHRQ Safety Program for Long-Term Care, use of a combined technical and socioadaptive intervention reduced CAUTI rates by 54% and culture orders by 15%.Reference Mody, Greene and Meddings124
The CDC recently funded a large-scale CAUTI intervention in medical-surgical units. This approach used 2 levels (or tiers) of interventions to approach and reduce persistently elevated CAUTI rates (Table 5 and Fig. 2). In short, the tier 1 interventions are applicable to all situations, regardless of CAUTI rates. However, if CAUTI rates remain elevated after implementing all 5 steps of tier 1 interventions, then the next tier is used. The process in tier 2 begins by using the Guide to Patient Safety, Reference Fletcher, Tyszka, Harrod, Fowler, Saint and Krein125 a validated tool developed to help HCP through the process of identifying barriers to reducing CAUTI rates. This tool uses a series of questions designed to help an organization identify areas for improvement coupled with evidence-based annotated responses to simple “yes or no” questions (Table 5 and Fig. 3; https://www.catheterout.org/cauti-gps.html).Reference Saint, Meddings and Fowler126

Figure 2. Infectious and noninfectious complications of an indwelling urethral catheter.
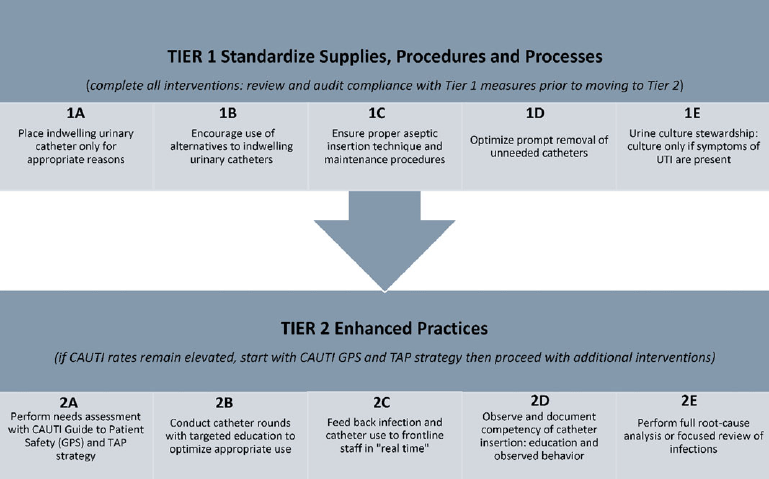
Figure 3. Tiered approach to reducing CAUTI.
Additional technical processes are often needed for successful implementation. However, technical processes alone rarely are enough to effect change. Those processes must also be adapted to specific social aspects of a given organization. This distinction between technical and socioadaptive (or behavioral) strategies is critical to proper integration of new processes.
Through social adaptation of technical strategies, innovation can be diffused in an organization.Reference Sreeramoju127 Consider technical means to reduce infection (eg, ready availability of hand sanitizers or bladder scanners and automatic urinary catheter stop orders in the hospital’s electronic health record system) as well as social adaptations such as changing organizational culture or norms of clinical practice or engaging clinicians with CAUTI initiatives. During a recent survey, Greene et alReference Greene, Gilmartin and Saint128 collected data from infection preventionists across the United States. Part of the survey evaluated how feelings of psychological safety interact with patient safety goals. Psychological safety is defined as “the degree to which people view the environment as conducive to interpersonally risky behaviors like speaking up if they witness an error or asking for help if they have concerns about an order.”Reference Greene, Gilmartin and Saint128 As an example of such a connection, they calculated the odds of regularly using technical initiatives for CAUTI reduction (eg, urinary catheter reminders) based on the degree of psychological safety the respondent felt in their organization. These researchers found high psychological safety (38% of respondents) to be associated with higher odds of using the technical initiatives (odds ratio, 2.37; P = .002).
Ensuring high psychological safety among HCP should also ideally be a foundational aspect of infection prevention practices. Similarly, survey results examining followership characteristics—personal attributes of those who follow a mentor, such as being energized by work, taking initiative, or independent thinking—demonstrated that the quality of follower has a direct influence on the uptake of recommended infection prevention practices.Reference Greene and Saint129 Frameworks like the Consolidated Framework for Implementation Research (CFIR) provide constructs across 5 domains: inner setting, outer setting, intervention, individuals, and process.130 These constructs provide a practical guide for assessing socioadaptive and technical aspects, diffusion of innovation, and program evaluation.
Investigators have also employed formal qualitative approaches to assess CAUTI implementation successes and challenges. For example, Krein et alReference Krein, Kowalski, Harrod, Forman and Saint131 examined the statewide impact of a “Bladder Bundle” initiative to reduce unnecessary use of urinary catheters. The team gathered qualitative data through semistructured interviews and site visits, resulting in the identification of key barriers to successful CAUTI reduction: (1) lack of nurse and/or physician engagement in the program; (2) patient and/or family requests for catheters; and (3) emergency department (ED) customs and practices.Reference Krein, Kowalski, Harrod, Forman and Saint131 Although these barriers represent important impediments, the investigators also identified strategies the participating hospitals used to address those barriers: (1) using urinary management (eg, planned toileting) in combination with other patient safety programs like fall prevention; (2) conversations with patients and/or families to clearly explain the risks associated with catheters; and (3) standardizing appropriateness criteria for staff working in the ED (to prevent placement of catheters for inappropriate reasons).Reference Krein, Kowalski, Harrod, Forman and Saint131 In another qualitative study, Harrod et alReference Harrod, Kowalski, Saint, Forman and Krein132 examined the perception of risk of HCP and how that related to their use of infection prevention practices such as indwelling urethral catheter. These study findings indicated that patient risk is not the only consideration for these HCP when deciding whether to use a urinary catheter. The study identified several areas for potential improvement: (1) the need for HCP to deal with competing priorities and insufficient time; (2) identifying “gray areas” where the connection cannot be directly made between the use of a urinary catheter and a negative patient outcome; (3) process weakness in either or both existing organization policies and the new initiative being undertaken; and (4) HCP using “workarounds” to undermine the effectiveness of catheter use reduction programs.Reference Harrod, Kowalski, Saint, Forman and Krein132 Investigators can thus tailor future interventions to address broader issues of risk to patient safety, realizing that objective risk from device use itself is not the only factor HCP consider when deciding to use a urinary catheter.
Implementation models like Capability–Opportunity–Motivation–Behavior (COM-B), Theoretical Domains Framework (TDF), and the Health Belief Model can be employed for better understanding of barriers and facilitators of CAUTI Prevention. Parker et alReference Parker, Giles, King and Bantawa133 also examined the barriers to and facilitators of a successful CAUTI reduction program by conducting 8 focus groups with a total of 35 individuals. Their team identified several major themes based on the use of their “NO CAUTI” bundle.Reference Parker, Giles and Graham134 They identified 2 facilitators to this process: (1) early and sustained key stakeholder engagement and (2) effective advance planning that allows for adaptation during implementation. They also reported 2 barriers: (1) managing the change itself is a burden and (2) sustaining practice change is difficult and must be properly managed.Reference Parker, Giles, King and Bantawa133 An overview of practical approaches for problem solving regarding potential barriers to implementation is provided in the Supplementary Content, Appendix 5 (online).
Finally, a key aspect of any intervention to reduce CAUTI (or other HAIs) is how sustainable the effects are. At the VA Ann Arbor Healthcare System, for example, a team implemented an intervention to reduce use of unnecessary urinary catheters (and thus reduce UTI incidence) in 2010.Reference Miller, Krein and Fowler135 This intervention significantly reduced catheter use on medical-surgical wards by 4.6% (absolute difference).Reference Miller, Krein and Fowler135 Fowler et alReference Fowler, Krein, Ratz, Zawol and Saint136 followed-up 8 years after this intervention, finding that the catheter prevalence had decreased from 13% to 25% (depending on unit type) to 7% (all units). Logistic regression of the data over the entire period (September 2010 to September 2019) indicated that catheter use decreased following intervention (odds ratio, 0.91; 95% CI, 0.86–0.97; P = .003). Inappropriate uses of catheters remained low during follow-up and did not appear to be affected by the intervention.Reference Fowler, Krein, Ratz, Zawol and Saint136 Similarly, Reynolds et alReference Reynolds, Sova and Lewis137 described a multifaceted CAUTI prevention initiative led by physician and nurse champions in ICUs at Duke University Hospital. The investigators observed a sustained reduction in rates of urine-culture utilization, catheter utilization, and CAUTI over 4 years. Ideally, more studies will assess long-term sustainability when implementing CAUTI prevention initiatives.
The 4-E’s model initially developed by Pronovost et alReference Pronovost, Berenholtz and Needham118 to reduce central-line–associated bloodstream infection can also be useful with efforts to reduce CAUTI. Here, using the 4 E’s model, we have outlined the steps that hospitals can use to implement CAUTI reduction programs.
Engage
Quality improvement projects directed toward improving compliance with CAUTI guidelines have used various techniques to engage the hospital staff to raise awareness of the issue and to increase buy-in.
-
1. Develop a multidisciplinary team:
-
a. Physician ledReference Elpern, Killeen, Ketchem, Wiley, Patel and Lateef138
-
b. Nurse ledReference Elpern, Killeen, Ketchem, Wiley, Patel and Lateef138–Reference Wenger140
-
c. Leadership not specified.Reference Wenger140–Reference Huang, Wann and Lin146
-
-
2. Involve local champions to promote the program.Reference Reynolds, Sova and Lewis137,Reference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139,Reference Oman, Makic and Fink145,Reference Fakih, Dueweke and Meisner147,Reference Venkatram, Rachmale and Kanna148
-
3. Utilize peer networking.Reference Meddings, Skolarus and Fowler66,Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144,Reference Venkatram, Rachmale and Kanna148,Reference Rosenthal, Ramachandran and Duenas149
-
4. Involve patient and family.
Educate
Education of the hospital staff can include in-person sessions or educational material available in paper format or electronically. The educational sessions may outline the evidence behind guidelines and the goals of the program and may target specific aspects of CAUTI prevention.
-
1. Provide education on the following topics:
-
a. Appropriate catheter careReference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139–Reference Titsworth, Hester and Correia141,Reference Oman, Makic and Fink145,Reference Rosenthal, Ramachandran and Duenas149–Reference Miller, Norris and Jenkins153
-
b. Appropriate indications for catheter insertion Reference Elpern, Killeen, Ketchem, Wiley, Patel and Lateef138,Reference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139,Reference Rhodes, McVay, Harrington, Luquire, Winter and Helms143,Reference Fakih, Dueweke and Meisner147,Reference Rosenthal, Ramachandran and Villamil-Gomez151,Reference Menegueti, Ciol and Bellissimo-Rodrigues154,Reference Shimoni, Rodrig, Kamma and Froom155
-
c. Insertion techniqueReference Titsworth, Hester and Correia141,Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144,Reference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151–Reference Menegueti, Ciol and Bellissimo-Rodrigues154
-
d. Urine-culture indications, guidance on collection, storage, and transport of urine cultures
-
e. Hand hygiene educationReference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151,Reference Winter, Helms, Harrington, Luquire, McVay and Rhodes152
-
f. Alternatives for indwelling catheters, including to patient and familyReference Meddings, Skolarus and Fowler66,Reference Oman, Makic and Fink145,Reference Topal, Conklin, Camp, Morris, Balcezak and Herbert156,Reference Schweiger, Kuster and Maag157
-
g. Management of urinary retention
-
h. Patient transportation.Reference Meddings, Skolarus and Fowler66
-
-
2. Provide educational materials as follows:
-
a. Daily assessment of need for catheterReference Fakih, Dueweke and Meisner147,Reference Narula, Lillemoe and Caudle158,159
-
b. Decision-making algorithm for catheter indication and urine-culture orderingReference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139
-
c. Case-based education by the infection prevention or stewardship teamReference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139,Reference Seidelman, Turner and Wrenn160
-
d. Unit-based educational materialsReference Misset, Timsit and Dumay161
-
e. Online learning materialsReference Van Decker, Bosch and Murphy61,Reference Oman, Makic and Fink145
-
f. Novel cognitive aids—screensaver, hospital leadership memorandumReference Luu, Dominguez and Yeshoua29
-
g. Patient and family educational materialsReference Oman, Makic and Fink145
-
h. Checklists for resident physicians162
-
i. Simulation training on catheter insertion and maintenance.Reference Van Decker, Bosch and Murphy61
-
Execute
The process for making quality improvement changes employs new protocols and algorithms. Interventions may be grouped into “bundles” or “checklists” of practices to be implemented simultaneously. The electronic medical record can be leveraged to prompt change in practices. Given the emphasis on quality improvement in physician training programs, engaging resident physicians and other learners in CAUTI prevention efforts may be helpful at teaching hospitals.
-
1. Standardize care processes as follows:
-
a. Perform daily assessments of the continued need for urinary catheterization.Reference Schweiger, Kuster and Maag157
-
b. Provide daily nursing or EMR reminders to physicians to remove unnecessary catheters, often via bedside rounds.Reference Van Decker, Bosch and Murphy61,Reference Elpern, Killeen, Ketchem, Wiley, Patel and Lateef138,Reference Rhodes, McVay, Harrington, Luquire, Winter and Helms143,Reference Oman, Makic and Fink145–Reference Fakih, Dueweke and Meisner147,Reference Apisarnthanarak, Thongphubeth and Sirinvaravong163–Reference Gao, Datta and Dunne165
-
c. Standardize indications for urinary catheter placement.Reference Elpern, Killeen, Ketchem, Wiley, Patel and Lateef138,Reference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139,Reference Titsworth, Hester and Correia141,Reference Dumigan, Kohan, Reed, Jekel and Fikrig142,Reference Fakih, Dueweke and Meisner147,Reference Schweiger, Kuster and Maag157,Reference Apisarnthanarak, Thongphubeth and Sirinvaravong163
-
d. Utilize bladder bundle.Reference Titsworth, Hester and Correia141,Reference Venkatram, Rachmale and Kanna148,Reference Winter, Helms, Harrington, Luquire, McVay and Rhodes152,Reference Misset, Timsit and Dumay161,Reference Jain, Miller, Belt, King and Berwick166,Reference Jaggi and Sissodia167
-
e. Develop a nurse-driven protocol to discontinue catheter if no longer meeting criteria.Reference Mitchell, Fasugba and Cheng96,Reference Wenger140–Reference Dumigan, Kohan, Reed, Jekel and Fikrig142,Reference Topal, Conklin, Camp, Morris, Balcezak and Herbert156,Reference Tyson, Campbell and Spangler168
-
f. Optimize the EMR with best-practice order sets, algorithmic decision making for catheter placement, urine cultures.Reference Youngerman, Salmasian and Carter169
-
-
2. Create reminders that the catheter is in place:
-
a. Alerts in EMR that a catheter has been placed (Banner, progress note templates)Reference Topal, Conklin, Camp, Morris, Balcezak and Herbert156,Reference Youngerman, Salmasian and Carter169
-
b. Alerts in EMR to remove cathetersReference Topal, Conklin, Camp, Morris, Balcezak and Herbert156,Reference Youngerman, Salmasian and Carter169
-
c. Use cognitive aids that specify line day.Reference Au, Shurraw, Hoang, Wang and Wang170
-
-
3. Appoint a resident quality champion (in teaching hospitals).Reference Bell, Alaestante and Finch171
-
4. Use prewritten or computerized stop orders.Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144,Reference Bell, Alaestante and Finch171,Reference Loeb, Hunt, O’Halloran, Carusone, Dafoe and Walter172
-
5. Utilize bladder scanners to measure urine volume prior to inserting a catheter.Reference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139,Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144,Reference Oman, Makic and Fink145,Reference Topal, Conklin, Camp, Morris, Balcezak and Herbert156
-
6. Standardize products and processes (catheter kit, alternatives to catheters, etc, or catheter maintenance processes).Reference Wenger140,Reference Titsworth, Hester and Correia141,Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144–Reference Huang, Wann and Lin146
-
7. Increase availability of bedside commodes.Reference Oman, Makic and Fink145
-
8. Conduct individual case reviews (or root-cause analysis) with interprofessional team to identify system issues and practice gaps.Reference Titsworth, Hester and Correia141
-
9. Create redundancy of educational materials using the following tools:
-
a. Posters, screensavers in unitsReference Reilly, Sullivan, Ninni, Fochesto, Williams and Fetherman139,Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144
-
b. Pocket cards, apps.Reference van den Broek, Wille, van Benthem, Perenboom, van den Akker-van Marle and Niel-Weise144
-
Evaluate
The success of a CAUTI quality improvement program can be measured by process, outcome, and balancing measures. Most programs have found that providing feedback to the hospital or unit increases awareness.
-
1. Measure performance or process:
-
a. Compliance with bundleReference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151,Reference Jaggi and Sissodia167
-
b. Compliance with hand hygieneReference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151,Reference Misset, Timsit and Dumay161,Reference Jaggi and Sissodia167
-
c. Urine culture utilization (overall rates of urine culture orders)
-
d. Catheter utilization (eg: catheter days, SUR, DUR).Reference Pepe, Maloney and Leung108
-
-
2. Provide real-time and routine feedback to staff and leadership:
-
a. CAUTI rates by wardReference Wenger140,Reference Goetz, Kedzuf, Wagener and Muder173
-
b. CAUTI rate by hospitalReference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151,Reference Apisarnthanarak, Thongphubeth and Sirinvaravong163
-
c. Hand hygiene rateReference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151
-
d. Catheter utilization (eg, catheter days, SUR, DUR)Reference Pepe, Maloney and Leung108
-
e. Catheter care complianceReference Rosenthal, Ramachandran and Duenas149,Reference Rosenthal, Ramachandran and Villamil-Gomez151
-
f. Costs of UTI.Reference Apisarnthanarak, Thongphubeth and Sirinvaravong163,Reference Kennedy, Greene and Saint174–Reference Hansen, Valentine-King and Zoorob178
-
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2023.137
Acknowledgements
The authors thank Jason Engle, MPH, for his help with optimizing the manuscript and graphics as well as reference management. The authors thank Valerie Deloney, MBA, for her organizational expertise in the development of this manuscript and Janet Waters, MLS, BSN, RN, for her expertise in developing the strategy used for the literature search that informs this manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the United States Department of Veterans’ Affairs.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
The following disclosures reflect what has been reported to SHEA. To provide thorough transparency, SHEA requires full disclosure of all relationships, regardless of relevancy to the topic. Such relationships as potential conflicts of interest are evaluated in a review process that includes assessment by the SHEA Conflict of Interest Committee and may include the Board of Trustees and the Editor of Infection Control and Hospital Epidemiology. The assessment of disclosed relationships for possible conflicts of interest has been based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The authors disclose the following: S.A. reports consulting fees with IDSA, bioMerieux, Locus Biosciences, and GSK, and participation on a bioMerieux Advisory Board, and is a co-owner of IPEC Experts, LLC. J.M. reports grant funding from the AHRQ, the CDC, the Ralph E. Wilson Foundation, and the VA HSR&D. All other authors report no conflicts of interest related to this article.