Purpose
Previously published guidelines provided comprehensive recommendations for detecting and preventing healthcare-associated infections (HAIs). The intent of this document is to highlight practical recommendations in a concise format designed to assist acute-care hospitals in implementing and prioritizing efforts to prevent HAIs through hand hygiene. This document updates the Strategies to Prevent Healthcare-Associated Infections through Hand Hygiene, published in 2014. This expert guidance document is sponsored by the SHEA. It is the product of a collaborative effort led by the SHEA, the Infectious Diseases Society of America (IDSA), the Association for Professionals in Infection Control and Epidemiology (APIC), the American Hospital Association (AHA), and The Joint Commission, with major contributions from representatives of organizations and societies with content expertise.
Summary of major changes
This section lists major changes from the Strategies to Prevent Healthcare-Associated Infections Through Hand Hygiene: 2014 Update, including recommendations that have been added, removed, or altered. Recommendations in this document are categorized as “essential practices” that are foundational to all HAI programs in acute-care hospitals. In 2014, these were “basic practices,” renamed to highlight their importance as foundational for hospitals’ healthcare-associated infection (HAI) prevention programs. Some recommendations are “additional approaches” that may be considered for use in locations and/or populations within hospitals during outbreaks or when HAIs are not controlled after implementation of essential practices. In 2014, these were “special approaches.” A complete summary of the recommendations contained in this document is provided in Table 1.
Table 1. Summary of Recommendations to Prevent Healthcare-Associated Infections through Hand Hygiene

Note. ABHS, alcohol-based hand sanitizer; CDC, US Centers for Disease Control and Prevention; WHO, World Health Organization; EPA, US Environmental Protection Agency; HCP, healthcare personnel.
Essential Practices
This section highlights updated recommendations based on evidence that was not available for consideration in the 2014 Compendium. There are 7 essential practices, and 5 of these are previously recommended practices with updated elements. However, 2 practices are new: glove use and prevention of environmental contamination.
The recommendation for promotion of healthy skin and fingernails is reinforced by high-quality evidence and emphasizes the preferential use of alcohol-based hand sanitizer (ABHS) in most clinical situations, which has been shown to be superior to handwashing in preserving healthcare personnel (HCP) hand skin integrity.
-
1. The document states that policies regarding the use of fingernail polish and gel shellac is at the discretion of the infection prevention program, except among HCP who scrub for surgical procedures, for whom fingernail polish and gel shellac should be prohibited.
-
2. The document recommends that facilities that are seeking ABHS with ingredients that may enhance efficacy against organisms anticipated to be less susceptible to biocides should consider manufacturers’ product-specific data.
-
3. The recommendation for placement of ABHS dispensers emphasizes unambiguous and visible accessibility within the workflow of HCP.
-
4. The document provides minimum thresholds for dispensers to ensure the accessibility of hand hygiene supplies.
-
5. The document contains additional recommendations for appropriate glove use:
-
a. HCP should receive competency-based training to ensure knowledge and skill in avoiding contamination during doffing.
-
b. Routine double-gloving is not recommended, except when specifically recommended for certain job roles or in response to certain high-consequence pathogens.
-
-
6. The document contains additional recommendations to reduce environmental contamination associated with handwashing sinks and sink drains.
-
7. Methods for monitoring adherence to hand hygiene now include direct overt observation, direct covert observation, automated hand hygiene monitoring systems, remote video observation, indirect measures, and audits of accessibility and functionality of supplies. Strengths and weaknesses of each method are discussed in Section 2 and listed in Table 4.
Additional Approaches
The document maintains the recommendation to wash hands with soap and water during outbreaks of C. difficile and norovirus but specifies that ABHS should not be prohibited when caring for patients with C. difficile or norovirus. During outbreaks of pathogens of premise plumbing, facilities may consider using a US Environmental Protection Agency (EPA)-registered disinfectant with disinfectant claims against biofilms.
Unresolved Issues
The recommendation regarding routine use of alcohol-impregnated hand wipes by HCP is unresolved due to the lack of noninferiority data.
Intended Use
This document was developed following the process outlined in the Handbook for SHEA-Sponsored Guidelines and Expert Guidance Documents. 2 No guideline or expert guidance document can anticipate all clinical situations, and this document is not meant to be a substitute for individual clinical judgement by qualified professionals. This guidance includes methods for the measurement of hand hygiene adherence, maintenance of healthy hand skin and fingernails for HCP, efficacy, and effectiveness of ABHSs, concerns related to outbreaks of waterborne pathogens of premise plumbing, and tools for implementation. This update is applicable to acute-care settings, but the principles and practices described may be indicated in any healthcare setting, including long-term and ambulatory healthcare settings. Hand hygiene is a broad term that includes healthy hand skin and fingernails and methods to clean them: handwashes, scrubs, and rubs. When recommendations are specific to the use of soap and water, the terms “handwash” or “hand scrub” are used. When recommendations are specific to the use of ABHS, the terms “hand sanitizing” or “hand rubbing” are used.
This document is based on a synthesis of evidence, theoretical rationale, current practices, practical considerations, writing-group consensus, and consideration of potential harm, where applicable. A summary of the recommendations is provided in Table 1.
Methods
The SHEA recruited 3 subject-matter experts in hand hygiene to lead the panel of members representing the Compendium partnering organizations: the SHEA, the Infectious Diseases Society of America (IDSA), the American Hospital Association (AHA), the Association for Professionals in Infection Control, and Epidemiology (APIC), and The Joint Commission, as well as the Centers for Disease Control and Prevention (CDC). The SHEA utilized a consultant medical librarian, who worked with the panel to develop a comprehensive search strategy for PubMed and Embase (January 2012–July 2019; updated in August 2021). Article abstracts were reviewed by panel members in a double-blind fashion through the abstract management software Covidence (Covidence, Melbourne, Australia). The articles were subsequently reviewed as full text. The Compendium Lead Authors group voted to update the literature findings, and the librarian reran the search to update it to August 2021. Panel members reviewed the abstracts of these articles via Covidence and incorporated relevant references.
Recommendations resulting from this literature review process were classified based on the quality of evidence and the balance between desirable and potential undesirable effects of various interventions (Table 2). Panel members met via video conference to discuss literature findings, recommendations, quality of evidence for these recommendations, and classification as essential practices, additional approaches, or unresolved issues. Panel members reviewed and approved the document and its recommendations.
Table 2. Quality of Evidence

Based on the CDC Healthcare Infection Control Practices Advisory Committee (HICPAC) “Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Recommendations Categorization Scheme for Infection Control and Prevention Guideline Recommendations” (October 2019), the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE), Reference Guyatt, Oxman and Vist156 and the Canadian Task Force on Preventive Health Care. 157
The Compendium Expert Panel, made up of members with broad healthcare epidemiology and infection prevention expertise, reviewed the draft manuscript after consensus had been reached by writing panel members. Following review and approval by the Expert Panel, the 5 partnering organizations, stakeholder organizations, and CDC reviewed the document. Prior to distribution, the guidance document was reviewed and approved by the SHEA Guidelines Committee, the IDSA Standards and Practice Guidelines Committee, and the Boards of SHEA, IDSA, APIC, and The Joint Commission. All members complied with SHEA and IDSA policies on conflict-of-interest disclosure.
Section 1. Rationale and statements of concern
Role of hand hygiene in acute care
Hand hygiene has long been a foundational component of infection prevention in all healthcare settings; however, adherence by healthcare personnel (HCP) to hand hygiene protocols has been an ongoing challenge, complicated by the lack of a national standard for measurement and increasingly complex care environments. Furthermore, the proliferation of hand hygiene products in recent decades has created challenges for healthcare administrators and infection prevention leaders to select the most effective, safe, and nonirritating products to support HCP hand hygiene. The purpose of this document is to provide practical guidance, based on up-to-date evidence, for decision making regarding implementation of hand hygiene programs in healthcare facilities.
In the years since publication of the Strategies to Prevent Healthcare-Associated Infections (HAI) through Hand Hygiene: 2014 Update, patients receiving healthcare have faced the ongoing threat of infections and antibiotic-resistant organisms potentially spread by contact with the hands of HCP. 3 Interaction with the healthcare environment can result in hand contamination following activities as brief as touching a bed rail. Reference Thom, Rock and Jackson4 Between 2013 and 2019, increases in numbers of patients colonized with extended-spectrum β-lactamase–producing Enterobacterales were noted, while the incidence of carbapenem-resistant Enterobacterales remained stable. 3 Healthcare facilities experienced the emergence of Candida auris, a resistant fungus, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus. Reference Schelenz, Hagen and Rhodes5,Reference Tsay, Kallen, Jackson, Chiller and Vallabhaneni6
State of hand hygiene in acute care
The coronavirus disease 2019 (COVID-19) pandemic was disruptive to infection prevention programs because it precipitated shortages in basic supplies, including ABHS. During the initial phase of the pandemic through December of 2021, the US Food and Drug Administration (FDA) enabled unprecedented use of locally produced ABHS through the provision of temporary guidance allowing for previously unregistered firms to manufacture ABHS. 7 The pandemic further strained hand hygiene programs because successful implementation relies on HCP input and engagement, which proved challenging throughout a protracted pandemic that resulted in staffing shortages and chronic stress incurred by HCP working in overburdened healthcare systems.
Estimating hand hygiene adherence in the United States is difficult given the variability in facility-specific methods for sampling and measurement. For example, in a point prevalence study conducted in a Canadian intensive care unit (ICU), adherence was reported as 83.5% among nurses and 45.2% among physicians. Reference Bruhwasser, Hinterberger and Mutschlechner8 In a study in a trauma resuscitation center in the United States, adherence was 7% overall and 0% before a clean procedure. Reference Haac, Rock and Harris9 In the latter study, if direct donning of gloves (ie, without hand hygiene) prior to a clean procedure was considered compliant, the adherence rate would have risen to 57% overall. Clearly there is room for improving adherence and ensuring that hand hygiene programs result in optimal adherence remains a critical element for preventing HAI.
Section 2. Background on the measurement of hand hygiene adherence
The goal of measurement
The goal of measuring hand hygiene is to provide timely, meaningful, and actionable feedback to guide HCP improvement. Elements of hand hygiene adherence that are amenable to measurement include the following: adherence to cleaning hands at the right moments before, during, and following care; evaluation of technique; the prevalence of hand dermatitis; and functionality and accessibility of equipment and supplies. Routine measurement should be performed to establish a performance baseline, to support improvement efforts, and to identify barriers and facilitators of adherence. It is unlikely that a single data-collection method will fulfill all the needs of a hand hygiene program.
Defining opportunities for hand hygiene
Indications for handwashing and hand sanitizing have been clearly defined. The United States Centers for Disease Control and Prevention (CDC) Core Infection and Control Practices for Safe Healthcare Delivery in All Settings, published in 2017, is now more closely aligned with the WHO My 5 Moments for Hand Hygiene in acute-care settings (Table 3). 10,11
Table 3. Indications for Hand Hygiene
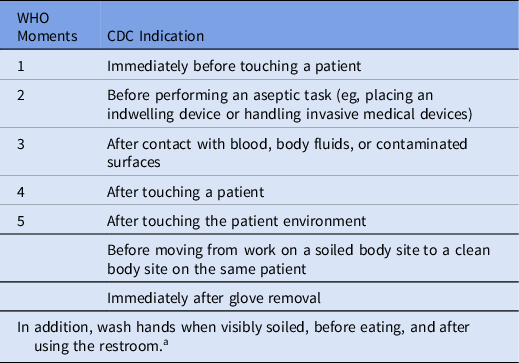
Note. WHO, World Health Organization; CDC, US Centers for Disease Control and Prevention.
a Hand sanitizing with an alcohol-based hand sanitizer is preferred unless handwashing is specifically indicated, or during outbreaks of C. difficile or norovirus.
Methods for measuring adherence
Healthcare facilities may choose from a variety of data collection methods to measure hand hygiene adherence: direct overt observation, direct covert observation, automated adherence hand hygiene monitoring systems (AHHMSs), remote video observation, patient as observer, indirect measurement via product usage, and audits of the functionality and accessibility of equipment and supplies. The measurement method used should be executed in a manner that enhances a culture of safety, results in credible and actionable data, and improves performance toward facility-specific goals. Personnel who conduct hand hygiene observations should be recognized as valued team members and patient safety advocates. Reference Arise, Nishizaki, Morita, Yagi and Takeuchi12,Reference Jain, Edgar and Bothe13 Some pitfalls of suboptimal execution of any measurement strategy include biased data, failures to improve adherence, and even the potential for workplace bullying of those collecting observations or reporting results. Reference Jain, Edgar and Bothe13,Reference Livorsi, Goedken and Sauder14 Strengths and weaknesses of hand hygiene measurement methods are summarized in Table 4.
Table 4. Methods to Measure Hand Hygiene
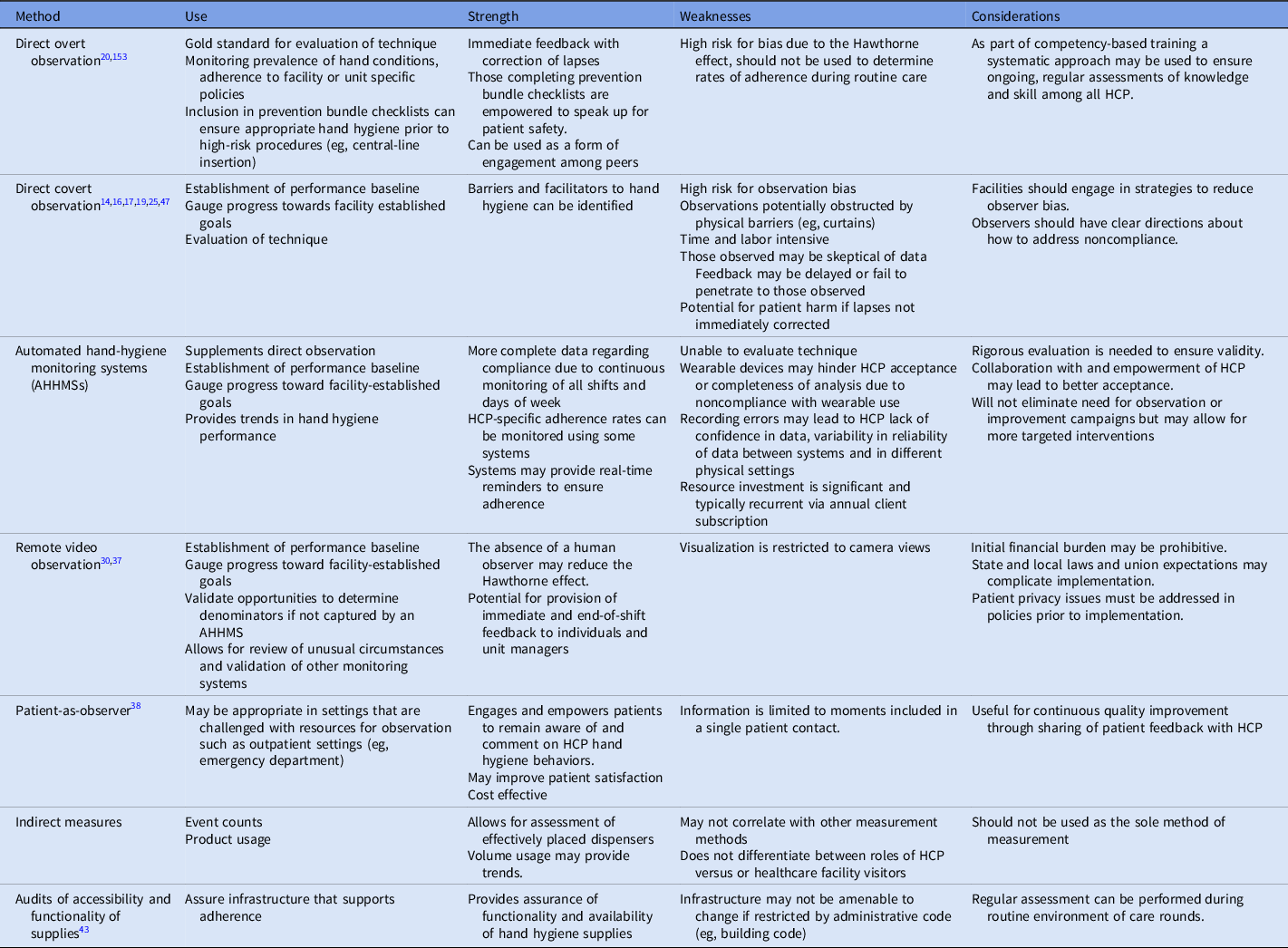
Direct overt observation
Direct overt observation (ie, the observer and the observed are known to one another) can be used to evaluate HCP hands for signs of dermatitis, adherence to facility-specific policies for fingernail length, and hand hygiene technique. When included in prevention bundles for high-risk procedures (eg, central-line insertion), direct overt observation can be used to provide immediate feedback, to correct lapses, and to ensure 100% adherence. Reference Korhonen, Ojanpera, Puhto, Jarvinen, Kejonen and Holopainen15 Because direct overt observation is inherently subject to bias driven by the Hawthorne effect (ie, deliberate changes in behavior based on the knowledge that one is being observed), it should not be used to determine hand cleaning adherence rates during routine patient care. Reference Baek, Kim, Kim, Cho, Hong and Kim16–Reference Wu, Lee and Chen20 Targeting direct overt observation to include certain HCP (eg, those performing high-risk procedures like central-line insertion) may be undertaken to ensure that all targeted personnel are observed.
Direct covert observation
Direct covert observation (ie, the observer is unknown to the observed) is also commonly referred to as a “secret shopper” or anonymous method. Although intended to reduce observer bias, multiple studies have documented observation and selection bias when those under observation became aware of the observer or when certain HCP, shifts, or areas in a unit or facility are oversampled. Reference McLaws and Kwok21 In a qualitative study of HCP in 10 acute-care hospitals, observers expressed concern about the Hawthorne effect, and those observed expressed concern about the inability of observers to see their compliance. Reference Livorsi, Goedken and Sauder14 In response to a survey about perceptions of hand hygiene monitoring, 58% of 1,120 British HCP did not strongly endorse direct observation for determining hand hygiene adherence. Reference Cawthorne and Cooke22
Methods to reduce bias and improve representativeness of observation include randomly and confidentially scheduling observations during peak activity (eg, morning rounds) and using a systematic method to determine where observations should be collected. Reference Fries, Segre, Thomas, Herman, Ellingson and Polgreen23 The specific times at which observed opportunities were found to be most representative of all opportunities in a medical ICU were 8 a.m., during morning rounds, 8 p.m., midnight, and 4 a.m. Reference Sunkesula, Meranda and Kundrapu24 To reduce the Hawthorne effect, facilities should consider increasing the frequency of observations, limiting observations to short periods of time (ie, 1–15 minutes), conducting unannounced audits, and enlisting observers unknown to unit personnel. Reference Scherer, Reisinger and Goto18,Reference Yin, Reisinger and Vander Weg25
Automated hand-hygiene monitoring systems (AHHMSs)
The use of AHHMSs has increased since the early 2000s. A variety of systems capture data in several different ways using event counters or wireless connections with dispensers and badges. Data may be collected in an aggregate form (ie, number of activations at room entry) or linked to specific personnel and specific indications. Measurement occurs on all shifts and all days of the week and captures large numbers of hand hygiene opportunities (HHOs), providing insight into adherence patterns. Reference Doll, Masroor and Cooper26,Reference Helder, van Goudoever, Hop, Brug and Kornelisse27 Accuracy may vary, and optimal methods of validating systems have not been identified. Reference Doll, Masroor and Cooper26,Reference Boyce, Cooper, Yin, Li and Arbogast28 Direct observation and remote video observation have been used to estimate denominator data for the calculation of adherence rates that may be used when an AHHMS does not capture HHOs. Reference Azim, Juergens, Hines and McLaws29–Reference Limper, Garcia-Houchins, Slawsky, Hershow and Landon32
Real or perceived data inaccuracies generated by AHHMSs may limit the ability to improve hand hygiene. Wearable devices that are unappealing, intrusive, or that interfere with hand hygiene (eg, wristbands) may lead HCP to reject use of the system. Reference Benudis, Stone and Sait33 Successful implementation of an AHHMS hinges on leadership commitment and collaboration among the infection prevention team and HCP. Reference Edmisten, Hall and Kernizan34 To enhance collaboration, one facility performed structured interviews to obtain HCP feedback about the use and functionality of the system. This intervention resulted in acceptance of the system and sustained improvements in hand hygiene adherence. Reference Al Salman, Hani, de Marcellis-Warin and Isa35 The cost of implementation of an AHHMS should be considered, including costs (eg, labor) related to other measurement methods.
Remote video observation
Remote video observation is a form of direct overt observation in that HCP and patients should be aware that cameras are viewing or recording their hand hygiene behavior. The observer is independent of the unit and unknown to those under observation, which reduces observer and sampling bias. Reference Brotfain, Livshiz-Riven and Gushansky36 Remote video observation has been used to validate and benchmark HHOs for use as denominators for certain AHHMSs. Reference Diller, Kelly, Blackhurst, Steed, Boeker and McElveen30 A study coupling remote video observation with rapid and regular feedback to HCP and unit managers resulted in sustained statistically significant improvements in 2 ICUs. Reference Armellino, Trivedi and Law37 To protect patient privacy the camera view may be restricted to areas of the room in which hand cleaning supplies are located and may have curtains that can be drawn over the lens. Reference Limper, Slawsky, Garcia-Houchins, Mehta, Hershow and Landon31 The major challenges of this method include the restricted view of the camera (ie, obstructed at times or limited to areas where hand hygiene is performed), the potential need for patient consent, and financial cost of systems.
Patient as observer
The patient as observer is another form of direct overt observation that may be useful in outpatient settings when resources for conducting hand hygiene observations are limited. An outpatient clinic using this method asked patients to complete a survey answering a single question, “Did your provider clean their hands before touching you?” More than 75% of patients returned the survey cards and expressed satisfaction with participation in the survey. During an evaluation phase, patient and nurse hand-hygiene auditing data were in concordance 86.7% of the time. HCP received regular individualized and aggregate adherence rates along with patient comments. Reference Le-Abuyen, Ng and Kim38 This strategy may have limited value in inpatient settings because of variations in patient ability to participate in observations and the increased number of HHOs associated with inpatient care.
Indirect measurement
Indirect methods of measuring adherence to hand hygiene include volume usage or event counters. Adherence to HHOs based on volume usage is often not attributable to specific department or units within a facility, limiting provision of feedback to HCP. When attempts were made to correlate usage with observational data, researchers noted high compliance by direct observation with no correlation to volume usage. This finding led them to suspect biased observations due to the Hawthorne effect. Reference Magnus, Marra and Camargo39,Reference Sodre da Costa, Neves and Marra40 Using an environmental assessment to identify points of care and an anticipated number of HHOs, indirect methods were used by one facility to determine that the facility would need 200,000 L of hand sanitizer annually to attain high rates of hand hygiene adherence. Reference Sicoli, Hunter, Shymanski, Suh and Roth41 This finding may not be helpful for measurement of adherence but may provide insight for emergency planning.
Audits of the accessibility and functionality of hand hygiene supplies
Regular audits of the accessibility and functionality of hand hygiene equipment and supplies should be conducted to ensure that HCP adherence is supported by the physical environment of care. The National Fire Protection Association requires that ABHS dispensers be tested for proper functioning each time they are refilled. 42 Audits of equipment can identify broken or empty dispensers or unsuitable sinks, which can then be remediated and tracked so that the facility continuously ensures that HCP have needed supplies to optimize hand hygiene adherence. Reference Cawthorne and Cooke22,Reference Bansaghi, Soule, Guitart, Pittet and Haidegger43
Sampling
No national standards have been established regarding the number of observations that should be conducted; however, processes of statistical analysis should be used to determine what constitutes an adequate and representative sample. Methods to estimate the total number of HHOs on various types of inpatient units have included direct observation and video surveillance. Reference Diller, Kelly, Blackhurst, Steed, Boeker and McElveen30,Reference Han, Conway and Moore44,Reference Sunkesula, Knighton, Zabarsky, Kundrapu, Higgins and Donskey45 Facility-wide, an AHHMS recorded between 1.5 and 2.5 million HHOs each month in a 400-bed hospital during the COVID-19 pandemic. Reference Makhni, Umscheid and Soo46 The number of HHOs is highest in ICUs (11.4 per patient hour) and lowest in mother–baby units (3.4 per patient hour). Reference Han, Conway and Moore44 The mean and median number of HHOs on medical and surgical units were 71.6 and 73.9 HHO per patient day, with a median of 46.7 HHOs on the first shift (7 a.m.–6:59 p.m.) and 28.0 on the second shift (7 p.m.–6:59 a.m.). Reference Diller, Kelly, Blackhurst, Steed, Boeker and McElveen30 When comparing compliance rates obtained by observing the WHO Five Moments to adherence upon entry and exit of patient rooms, a similar adherence rate was observed, indicating that sampling at entry and exit to the room may provide an adequate sample while also decreasing barriers to observation. Reference Sunkesula, Meranda and Kundrapu24,Reference Chang, Reisinger and Jesson47
Feedback of results
Feedback of measurement results is critical for performance improvement. Goal setting and immediate active feedback have been associated with improved adherence. Reference Diefenbacher, Fliss, Tatzel, Wenk and Keller48,Reference Storey, FitzGerald and Moore49 Feedback is most effective when provided by a supervisor or colleague, when it is provided more than once, when it is given verbally and in writing, and when it is associated with clear targets and action plans. Reference Ivers, Jamtvedt and Flottorp50 In a facility that aimed to improve adherence among physicians provided regular reports to chiefs of service, comparative rankings of service varied initially but rose to >90% each month, with sustained improvement over a 2-year period. Reference Reich, Goodstein and Callahan51
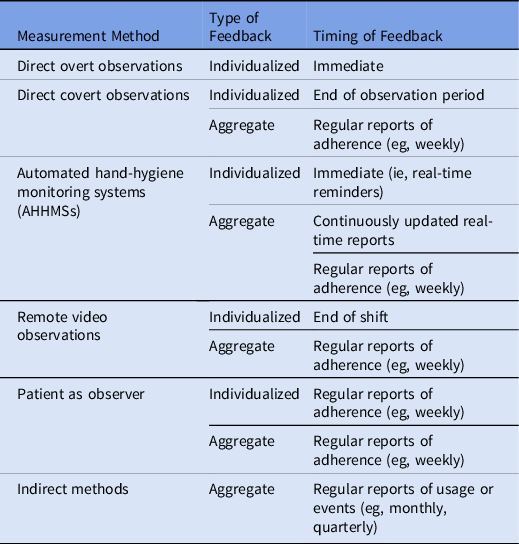
Feedback that fails to reach frontline personnel is a barrier to performance improvement. Reference Jain, Edgar and Bothe13,Reference Livorsi, Goedken and Sauder14 A variety of methods used to provide feedback have included aggregating data and displaying results visually in real time or at the end of a shift. When video feedback of their own performance was confidentially shared with HCP working in hemodialysis, together with written feedback, 7 of the 11 HCP included in the study demonstrated improvement in adherence. Reference Sánchez-Carrillo, Rodríguez-López and Galarza-Delgado52 To provide intuitive feedback, a visualization of a handprint with decreasing numbers of bacteria as performance improved, rather than a numeric adherence rate, was presented to HCP. This feedback method did not generate improved performance. Reference Scherer, Reisinger and Goto18 Observation methods are linked to timing of feedback in Table 5.
Table 5. Type and Timing of Feedback by Hand Hygiene Measurement Method
Section 3. Background on prevention of HAIs through hand hygiene
Summary of existing guidelines and recommendations
Guidelines for hand hygiene in healthcare settings have been published by the following organizations:
-
1. Healthcare Infection Prevention Practices Advisory Committee (HICPAC), Centers for Disease Control and Prevention (CDC) 53
-
2. The World Health Organization (WHO) 11
-
3. The Association for Perioperative Registered Nurses (AORN), related to perioperative hand hygiene 54
-
4. The Society for Healthcare Epidemiology of America (SHEA) related to hand hygiene for the operating room anesthesia work area. Reference Munoz-Price, Bowdle and Johnston55
Infrastructure requirements
Hand hygiene programs should have the following elements in place:
-
1. Accessible and functional hand hygiene supplies, including hand sanitizer dispensers with adequate supplies of ABHS, handwashing sinks, plain or antiseptic handwash, disposable or single-use towels, and hand moisturizer that is compatible with other products and gloves
-
2. Senior and unit-based leadership support that is responsible and accountable for ensuring engagement and adherence of frontline personnel
-
3. Infection prevention personnel with training and resources to direct programs aimed at improvement of hand hygiene
-
4. HCP who have received training to recognize indications for hand hygiene throughout care episodes
-
5. Trained observers to collect adequate observations to evaluate technique and monitor performance (If automated hand hygiene monitoring systems are used, those charged with their oversight should be skilled in validating data obtained from the system.)
-
6. Support for data analysis and meaningful communication of monitoring results regardless of the measurement method used by the facility.
Literature review
Healthy hand skin and fingernails
Hand hygiene begins with the healthy hands of HCP, defined as being free from pathogenic transient or resident flora, redness, cracks, or wounds, and having short, natural fingernails. 11 Natural fingernails and those with standard fingernail polish were shown in a single study to be more amenable to cleaning with ABHS than gel or shellac fingernails. Reference Hewlett, Hohenberger and Murphy56 No studies of artificial fingernails or chipped fingernail polish were identified, likely indicating acceptance of previous research related to their association with increased pathogenic flora. 54 Ongoing exposure to the healthcare environment, water, and antiseptics challenges the barrier integrity of HCP hand skin, placing them at high risk for occupational irritant and allergic contact dermatitis. Reference Behroozy and Keegel57 In the United Kingdom, 414 (15%) of 2,762 HCP surveyed indicated that their skin had suffered due to work. Reference Campion58 Self-reported symptoms of hand eczema were reported by 579 (47%) of 1,232 HCP surveyed in the Netherlands. Among those with hand eczema in the previous 3 months, 84% reported performing their regular duties at least 1 day while symptomatic. Reference van der Meer and Vandenbroucke-Grauls59
Allergens associated with hand hygiene include antiseptics, latex, rubber accelerators, fragrances, surfactants, and preservatives. Reference Rundle, Presley and Militello60 Increasing exposure of HCP to chlorhexidine gluconate (CHG), in both HCP hand hygiene products, and in patient bathing products has stimulated research examining the potential for sensitization of HCP to CHG. In a cross-sectional survey of >1,000 nurses, >70% reported using chlorhexidine >20 times per shift. Reference Barnes, Stuart and Redley61 Also, 114 (30.7%) of these nurses experienced symptoms of sensitization and regularly experienced self-reported symptoms included dry skin (86.7%), localized rash (73.3%), and wheezing or coughing (20.6%). No anaphylactic events were reported. Hand hygiene programs should implement strategies to engage HCP in primary and secondary prevention of hand eczema, and allergic or irritant dermatitis. Reference Clemmensen, Randboll, Ryborg, Ebbehoj and Agner62–Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64
Hand hygiene product safety and efficacy
Regulatory background
In December 2017, the FDA’s tentative final monograph for over-the-counter healthcare antiseptic drug products, initially published in 1994, and amended in 2015, was finalized. 65 This rule established active ingredients for over-the-counter use as HCP handwashes or surgical hand scrubs, HCP hand rubs and surgical hand rubs. Pending additional data to establish generally recognized as safe and effective (GRAS/GRAE) determinations, the FDA deferred regulatory action on 6 antiseptics included in the final monograph: benzalkonium chloride, chloroxylenol, ethyl alcohol, isopropyl alcohol, and povidone iodine. 65,66 The FDA requires manufacturers to meet current safety standards related to human safety, nonclinical safety (eg, reproductive, toxicity studies), potential hormonal effects, and antimicrobial resistance. Although still eligible for inclusion in HCP handwash, triclosan was removed from the consumer antiseptic monograph due to potential hormonal effects and potential contribution to antimicrobial resistance. 67 CHG was introduced in the United States after the 1994 tentative final monograph was published and was determined to be ineligible for inclusion; products formulated with CHG continue to be regulated as new drugs.
Safety
In response to the FDA request for up-to-date safety data, observational studies aimed at estimating maximal use of ABHS by HCP have been conducted. Studies using electronic event counting have reported wide variation in usage frequency depending on role and service (eg, nurse vs physician and operating room vs medical ICU). Nurses likely have the most frequent exposure to hand-sanitizing formulations, and the maximal use among 95% of nurses working 12-hour shifts has been reported as 15 uses per hour or 141 uses per shift. Reference Boyce, Polgreen, Monsalve, Macinga and Arbogast68
Potential for absorption of ABHS
Dermal absorption of alcohol has been considered a potential reproductive risk for women as there are no established minimum safe levels of alcohol exposure during pregnancy. Researchers conducting a safety assessment noted that ABHS can result in very low but detectable internal doses that approximate those associated with consumption of nonalcoholic beverages (eg, juices that may undergo natural fermentation). The authors concluded that the benefits of preventing infection by using ABHS outweigh risks of maternal exposure to alcohol through dermal absorption. Reference Maier, Ovesen and Allen69
Respiratory absorption of alcohol was examined among preterm neonates in isolettes. Volatilized ethanol from a single exposure resulted in a detectable level of blood alcohol (0.036 mg/dL), lower than the European Medicines Agency limits for ethanol exposure in children. Reference Hsieh, Sapkota, Wood, Bearer and Kapoor70 Exposures of neonates could be reduced by ensuring drying of ABHS prior to placing hands within an isolette. Evidence about developmental toxicity related to antiseptics other than alcohol is limited. Respiratory absorption of alcohol was also studied among anesthesiologists; 8 of 130 breathalyzer tests were positive within 2 minutes of use of ABHS, and none resulted in a positive blood alcohol reading. Reference Lindsay, Hannam, Bradfield and Mitchell71
Adverse events
The literature review did not yield any reports of serious harm associated with the use of ABHS in healthcare settings. Two cautionary reports from community settings may have relevance for healthcare facilities. The first involved failures in the manufacture of an ABHS produced outside the United States and distributed during the SARS-CoV-2 pandemic. An FDA consumer alert warning about hand sanitizers that contained methanol resulted in 2 states reporting 15 cases of methanol poisoning following intentional consumption. Methanol poisoning resulted in 4 deaths and permanent disabilities in survivors. Reference Yip, Bixler and Brooks72 In areas in which intentional consumption is an identified risk (eg, behavioral health units) measures to maintain control of ABHS should be taken.
The second harmful event occurred outside the United States and involved unsafe dispensing of ABHS. The height of the dispensers and the method of activation (eg, a foot pump) allowed ABHS to be directed toward the faces of small children, resulting in splashing of the eyes and severe ocular injuries. Reference Martin, Le Roux and Guindolet73,Reference Yangzes, Grewal, Gailson and Grewal74 In the United States, the National Fire Protection Association requires dispensers be designed so that accidental activation is minimized and requires that dispensers be tested each time a new refill is installed. This may be particularly important in pediatric facilities. 42 All local administrative codes related to fire safety when choosing locations for installation of ABHS dispensers and storage of refills should be followed. Pediatric facilities should evaluate the height of wall-mounted ABHS dispensers and the placement of pump bottles to avoid activation by young children, and adult supervision should be ensured.
Efficacy of hand hygiene formulations
The current approval process for antiseptic drug products does not allow manufacturers to make organism or disease-specific prevention claims, and results of efficacy studies may be difficult to compare. Premarket assurance of efficacy is determined using methods published by the American Society for Testing and Material (ASTM) or internationally using the European Norm. Reference Singal, Higgins and Waljee75 These methods generally evaluate bacterial log reduction on artificially contaminated hands or finger pads. Handwashes are tested using a 5-mL dose, and hand rubs are tested using a 1.5-mL dose or a single impregnated wipe. Test organisms are either gram-negative organisms (eg, Serratia marcescens and Escherichia coli) or gram-positive organisms (eg, Staphylococcus aureus). The ASTM recommends methods of efficacy testing against viruses and fungi, but these are not required prior to distribution in the United States. 11
Efficacy of ABHS
Viral pathogens
Prior to the pandemic, suspension testing evaluating the efficacy of WHO hand-rub formulations found that both formulations inactivated Ebola and emerging coronaviruses with a 30-second exposure time. Reference Siddharta, Pfaender and Vielle76 A study investigating efficacy against adenovirus serotypes 8, 19, and 37, typically associated with epidemic keratoconjunctivitis, reported a 2.5 log10 reduction with combinations of alcohol and lower reductions when alcohol was combined with CHG. Reference Uzuner, Karadenizli, Er and Osmani77
A systematic review of 56 studies testing efficacy of ethanol against viruses found high efficacy against enveloped viruses and less efficacy against nonenveloped viruses. Efficacy against nonenveloped viruses was improved when acids were added to alcohol-based formulations. Reference Kampf78 This finding is consistent with other studies showing that excipient ingredients (ie, those other than the active ingredient) can enhance or reduce efficacy of alcohol such that in certain formulations, lower volumes of ethanol may produce higher reductions in bacteria than formulations with higher ethanol concentrations. Reference Edmonds, Macinga and Mays-Suko79
Candida auris
A study examining germicidal activity of hand-sanitizing preparations against C. auris demonstrated that a 70% ethanol-based hand sanitizer resulted in a 4 log10 reduction in organism when tested using a quantitative carrier method. Reference Fu, Le and Liu80 Surgical hand scrubs containing CHG resulted in <2.0 log10 reduction and were less efficacious when alcohol was not included in the formulation. Researchers also investigated 2 alcohol-based formulations: a combination of ethanol (54%–66%)–isopropyl (9%–11%) and a 75% ethanol sanitizer against C. auris on artificially contaminated pig skin. The formulations reduced organism load by 2.92 log10 and 2.44 log10, respectively. Reference Fu, Le and Liu80
Vancomycin-resistant Enterococcus (VRE)
Isolates from a single healthcare institution exhibited tolerance when exposed to 23% (v/v) isopropanol. Reference Pidot, Gao and Buultjens81 When these isolates were exposed to isopropyl alcohol at 70% (v/v) in a broth culture, complete killing and an 8 log10 reduction was obtained. These researchers hypothesized that as tolerance increases exposure of E. faecium to less than the maximum biocide concentration could select for increasing tolerance. These findings emphasize the importance of adequate formulations and appropriate real-world applications.
Efficacy of benzalkonium chloride
The persistent activity of benzalkonium chloride (BK), a quaternary-ammonium compound, either alone or in combination with alcohol, has been described. When S. aureus was used as test organism, BK alone produced log10 reductions up to 4 hours after application. Reference Herruzo, Yela and Vizcaino82,Reference Bondurant, Duley and Harbell83 BK 0.2% produced >3 log10 reductions in SARS-CoV-2 within 15 seconds of exposure. Reference Ogilvie, Solis-Leal, Lopez, Poole, Robison and Berges84 Evidence against gram-negative organisms is lacking and concerns remain about the intrinsic resistance of Burkholderia cepacia complex to BK. Reference Ahn, Kim and Kweon85 Due to the organism’s ability to adapt to nutrient-depleted solutions, B. cepacia complex poses a risk for contamination of non–alcohol-based hand sanitizers. Reference Tavares, Kozak and Balola86 In 2020, a recall of non–alcohol-based hand sanitizer followed product contamination with B. cepacia complex from the municipal water supply used in production. 87 Non–alcohol-based hand sanitizers should not be used in clinical settings.
Effectiveness in clinical use
Real-world hand-hygiene effectiveness is related to product formulation, application volume, thorough application to all hand surfaces, and rates of personnel adherence. Reference Goldstein, Eppes, Mackley, Tuttle and Paul88–Reference Sickbert-Bennett, DiBiase, Willis, Wolak, Weber and Rutala90 In a network modeling study describing methicillin-resistant S. aureus (MRSA) colonization rates among neonatal ICU (NICU) patients, colonization was reduced as hand hygiene adherence increased. Reference Goldstein, Eppes, Mackley, Tuttle and Paul88 Even under optimal conditions, the most vulnerable patients may still acquire pathogens; therefore, HCP should aim for high adherence to each element of hand hygiene.
A randomized control trial examined the effectiveness of 3 hand hygiene protocols comparing ABHS application to all hand surfaces, ABHS application using a WHO-recommended structured hand rub and chlorhexidine gluconate (CHG) handwash. ABHS was as effective as the CHG handwash in reducing bacteria on the hands, and ABHS application was the most time-efficient means of performing hand hygiene. Reference Chow, Arah and Chan91 This study was replicated using MRSA as a test agent, and ABHS was as effective as CHG handwash in real-world use. Reference Ho, Poh, Choudhury, Krishnan, Ang and Chow92 Residual effectiveness has been demonstrated with formulations that combine alcohol with either 2%–5% CHG or 0.1% BK. Reference Herruzo, Yela and Vizcaino82 Such formulations may be beneficial particularly if used in high-risk areas (eg, ICU or transplant units) or prior to invasive procedures like central-venous access.
Mode of delivery
ABHS is available in several delivery forms such as liquid, gel, foams, and wipes. Alcohol-impregnated wipes were previously reported to have similar efficacy to gel and foam hand rubs when influenza virus was the organism of interest. Reference Larson, Cohen and Baxter93 In a study using E. coli as the test organism to compare ABHS hand rubs to cotton or polypropylene hand wipes, hand rubs were superior to hand wipes. Reference Ory, Zingg, de Kraker, Soule and Pittet94 Further testing is needed to determine noninferiority of alcohol-impregnated hand wipes to hand rubbing with ABHS.
Effective volume and dose
The volume of hand sanitizer or antimicrobial handwash formulations may be considered a dose, and the dose must be sufficient to cover all surfaces of the hands. Touch-free dispensers provide a mean dose ranging from 0.6 mL to 1.3 mL with a mean drying time of 12–22 seconds. Reference Macinga, Edmonds, Campbell, Shumaker and Arbogast89 For persons with large hands, a dose of 4–6 mL may be needed to achieve >2 log10 reductions in bacteria on the hands. Reference Bellissimo-Rodrigues, Soule, Gayet-Ageron, Martin and Pittet95 To obtain antisepsis, the volume of ABHS should be customized according to the size of the individual’s hands. This volume may be communicated as a “palmful” of hand sanitizer and may require more than a single activation. Some AHMMSs measure only 1 activation if multiple dispenser activations occur during brief time spans (eg, 2 seconds), which may be important in avoiding the overestimation of compliance if event counters are used for measurement.
Effective technique
Techniques for both handwashing and hand sanitizing should focus on coverage of all hand surfaces for an appropriate length of time. This literature review did not identify studies examining the duration of handwashing; a minimum of 15 seconds of scrubbing for routine handwashing has been previously recommended by the CDC. 53 Studies have reported no difference in bacterial load when comparing hand-rub durations of 15 and 30 seconds, but adherence increased by 27% with shorter hand rubs. Reference Harnoss, Dancer and Kaden96,Reference Pires, Soule, Bellissimo-Rodrigues, Gayet-Ageron and Pittet97 When technique was included with observations of adherence to indications for hand hygiene, only 7% of HCP attained full coverage of all hand surfaces; the thumb and fingertips were the most frequently missed areas of the hands. Reference Park, Kim and Lim98 Attainment of full hand-surface coverage while rubbing for 15 seconds or longer should be included in HCP evaluations of hand-hygiene technique. Reference Harnoss, Dancer and Kaden96,Reference Pires, Soule, Bellissimo-Rodrigues, Gayet-Ageron and Pittet97
Organisms with less susceptibility to biocides
Spore-forming organisms (eg, C. difficile and B. cereus) and small nonenveloped viruses (eg, norovirus) are difficult to inactivate with surface disinfectants and may not be inactivated by alcohol. Many facilities lack clarity regarding whether alcohol-based hand sanitizer should be used when contact with organisms that are less susceptible to biocides occurs. These organisms may also be difficult to remove through handwashing. Reference Kampf and Kramer99 Using a nontoxigenic strain of C. difficile to test reductions associated with handwashing technique a 1.3 log10 reduction was attained with an unstructured handwash and a 1.7 log 10 reduction was attained using a structured handwash (ie, the WHO How to Handrub). Reference Deschenes, Chano, Dionne, Pittet and Longtin100
When exposure to potential spore-forming organisms or small nonenveloped viruses is anticipated (ie, patients diagnosed with C. difficile infection or norovirus or those with new acute diarrhea or vomiting), the CDC recommends standard and contact precautions for all contacts with the patient and their surroundings. HCP need clear messaging about hand hygiene in response to these organisms. Those directing hand hygiene programs should do the following: (1) help HCP remain aware of organisms with biocide resistance that are circulating in the facility; (2) emphasize the importance of reducing hand contamination through the use of gloves according to standard and contact precautions; (3) maintain the availability of ABHS in the presence of these organisms; (4) in all settings, regardless of organisms present, always wash hands if visibly soiled, before eating, and after using the bathroom; and (5) emphasize the importance of thorough hand cleaning with the consideration of educating HCP in WHO-structured techniques for handwashing and hand sanitizing. Reference Kirk, Kendall and Marx101
Accessible hand hygiene supplies
Among 350 HCP surveyed in the United States and Canada, lack of access to supplies was described as the primary barrier to adherence. Reference Kirk, Kendall and Marx101 The physical infrastructure required to implement hand hygiene in all facilities consists of access to ABHS and handwashing stations supplied with water, soap (ie, plain or with an antiseptic), towels, gloves, and hand moisturizers that are compatible with antiseptics and gloves. In perioperative and procedural areas, including ICUs, surgical hand scrub and surgical hand rub should also be accessible to HCP.
Supplies for hand sanitizing
Several studies have examined optimal placement of ABHS dispensers within HCP workflow. On a general inpatient unit, sequentially increasing the number of wall-mounted ABHS dispensers above a minimum threshold (defined as 2 dispensers per room, 1 dispenser in the hallway, and 1 dispenser in the patient room) did not result in improved adherence. Reference Chan, Homa and Kirkland102 More than half of hand hygiene events occurred in the hallway. Once inside the room 75% of events involved dispensers just inside the doorway. In multipatient rooms (eg, bays), a threshold for accessibility was considered 1 dispenser for every 2 beds. Reference Diefenbacher, Fliss, Tatzel, Wenk and Keller48 Ensuring accessibility to ABHS was most difficult when workflows involved crowded spaces with no dedicated bed space (eg, hallway care). In these spaces, a focus on the WHO Five Moments and the CDC indications prior to and immediately following patient care may be helpful. When patients cannot be housed in rooms, facilities should ensure that the patient zone is clearly defined and that hand hygiene supplies are within reach. Reference Carter, Wyer and Giglio103,Reference Salmon and McLaws104
In addition, ABHS dispensers that are clearly identifiable (ie, distinct from soap or moisturizer dispensers), function as visual cues to perform hand hygiene. Improved adherence to hand hygiene when ABHS dispensers are visible and accessible within the workflow of HCP has been replicated in multiple healthcare and specialty settings and among inpatient and outpatient areas. Reference Cure and Van Enk105–Reference Smolkin, Roth-Ahronson and Kranzler111 Event counters can be used to establish the best location for dispensers on individual units and within patient rooms. Reference Boog, Erasmus, de Graaf, van Beeck, Melles and van Beeck112 When units are well equipped with mounted dispensers, individual pocket-sized dispensers did not increase adherence to hand hygiene, possibly because individual-sized dispensers are more difficult to use than wall-mounted dispensers. Reference Keller, Wolfensberger and Clack113 When ABHS dispensers cannot be wall mounted, and there is no risk of intentional ingestion, pump bottles can be mounted on beds or placed on bedside tables, work surfaces, and other locations in the workflow of personnel.
Supplies for handwashing
Accessibility and visibility of sinks affects HCP adherence to handwashing. Sinks visible from the point of care, rather than sinks that are separated from the point-of-care by a wall or door, resulted in more frequent handwashing with longer duration, particularly if visible from occupied beds. Reference Cloutman-Green, Kalaycioglu and Wojani114 Following care of individuals with C. difficile infection, proper timing of glove removal upon leaving the patient zone was directly associated with hand washing, whereas increasing distance of the sink was inversely associated with handwashing compliance. Reference Deyneko, Cordeiro, Berlin, Ben-David, Perna and Longtin115
Contamination of water and plumbing
Contamination of supply or wastewater (ie, biofilms within sink drains) with waterborne pathogens of premise plumbing may increase opportunities for the environmental contamination of HCP hands, clothing, and patient care supplies. Reference Cloutman-Green, Kalaycioglu and Wojani114,Reference Balm, Salmon and Jureen116–Reference Roux, Aubier, Cochard, Quentin and van der Mee-Marquet123 Splashing of water and aerosolization of organisms has included extended-spectrum β-lactamase–resistant organisms, Enterobacterales, Elizabethkingia meningoseptica, KPC-2–producing Klebsiella spp, and Pseudomonas spp. Reference Balm, Salmon and Jureen116,Reference Leitner, Zarfel and Luxner120 In an observational study analyzing behavior at sinks in an ICU, handwashing occurred in only 4% of the total interactions with the sink. Other activities included filling and emptying of water glasses, medication cups, and tube feed bags, draining IV bags, preparation and discarding of food and beverages and placement of patient care items on nearby countertops. Reference Grabowski, Lobo and Gunnell124 Slow drainage from sinks was also noted to result in increased contamination of the sink bowl and nearby surfaces. Reference Hajar, Mana, Cadnum and Donskey118 Contamination was reduced when sink use was restricted to handwashing and basins were disinfected daily with bleach. Reference Roux, Aubier, Cochard, Quentin and van der Mee-Marquet123 In 2017, the United States Environmental Protection Agency released regulatory guidance for pesticidal claims against biofilm bacteria on hard, nonporous surfaces including sink drains. 125 As of 2022, several pesticides have been registered, although there are no protocols for use or optimal intervals for drain cleaning have been published. Handwashing sinks should be included in water infection control risk assessments. Resources are available on the CDC Reduce Risk from Water webpage. 126
Contamination of supplies
Contamination of soap and ABHS dispensers is less frequently described. However, ABHS dispensers have been found to be contaminated with Staphylococcal spp, this contamination increases with increasing use of the dispensers. Reference Cloutman-Green, Kalaycioglu and Wojani114 During the SARS- CoV-2 pandemic, the CDC advised against refilling (or “topping off”) dispensers intended for single use, acknowledging a lack of studies but the potential for introducing spore-forming organisms. 127 Higher rates of mechanical defects were reported among touchless ABHS dispensers compared to mechanical dispensers, suggesting the potential need for ongoing maintenance. Reference Roth, Batzer, Hug and Widmer128 Using a crossover design, researchers examined the use of jet air dryers compared with single-use towels. The target organisms (methicillin-susceptible S. aureus, methicillin-resistant S. aureus, enterococci, and extended-spectrum ß-lactamase–producing bacteria) were recovered from washroom floors and from the surfaces of jet air dryers. These researchers concluded that single-use towels had less propensity for environmental dispersion of organisms and that jet air dryers were not acceptable for clinical use. Reference Best, Parnell and Couturier129
Nonsterile glove use
Use of nonsterile gloves is inextricably linked to hand hygiene, not only providing benefits like reduced hand contamination during care but also introducing risks such as increased hand contamination during doffing and increased contamination of the patient care environment. Studies evaluating the transfer of environmental contaminants to gloves and bare hands have reported a reduction in hand contamination when gloves are worn. The microbial load of gloved and bare hands stabilized after 4–6 contacts within a patient environment; gloved hands having a microbial load 4.7% lower than bare hands. Reference King, López-García and Atedoghu130 Hand contamination increased when gloves fit poorly (ie, were too large), likely due to increased exposed surface area. Transfer efficiencies of A. baumannii when latex gloves were worn reduced fomite-to-fingerpad transfer by 56% and reduced fingerpad-to-fomite transfer by 47%. Reference Greene, Vadlamudi, Eisenberg, Foxman, Koopman and Xi131 As anticipated, failure to wear gloves was independently associated with hand contamination following care of patients with C. difficile infection. Reference Landelle, Verachten, Legrand, Girou, Barbut and Brun-Buisson132
In a randomized clinical trial studying the impact of nonsterile glove use after hand hygiene versus hand hygiene alone for all care, no significant difference was detected in late-onset invasive infection or necrotizing enterocolitis in neonates cared for by HCP in these 2 groups. Significantly fewer gram-positive bloodstream infections and central-line–associated bloodstream infections occurred among neonates whose care providers donned gloves after hand hygiene. Reference Kaufman, Blackman, Conaway and Sinkin133 This finding suggests that hand hygiene plus donning nonsterile gloves prior to patient and vascular device contact may lessen the risk of infection among units that care for preterm neonates.
Numerous barriers to hand hygiene prior to use of nonsterile gloves have been reported. Frequently observed noncompliant behavior includes reductions in hand hygiene prior to patient contact and failures to change gloves at appropriate moments. Reference Kuruno, Kasahara and Mikasa134 In a qualitative study, HCP reported that donning gloves on wet hands was unpleasant and that donning gloves without hand hygiene immediately prior saved time, particularly if anticipated contact was brief (eg, delivering a patient food tray). Physical barriers, such as lack of ABHS access at points where gloves are donned, also resulted in nonadherence. Reference Baloh, Thom and Perencevich135 In an ICU, when hand hygiene prior to donning gloves was compared to direct gloving (ie, no hand hygiene prior to donning nonsterile gloves), there was no significant difference in the average CFU on the surface of the gloves (6.9 vs 8.1 CFU, respectively). Reference Rock, Harris, Reich, Johnson and Thom136 It took an average of 31.5 extra seconds to perform hand hygiene before donning gloves, which equates to ∼20 minutes of extra time for the average ICU nurse caring for a patient in contact isolation during a 12-hour shift. Neither the CDC nor the WHO consider donning nonsterile gloves to be an indication for hand hygiene, but it is frequently associated with Moment 1 of the WHO My 5 Moments and the CDC indication for hand hygiene prior to patient contact. Infection preventionists and hospital epidemiologists should evaluate the potential impact to patient and HCP safety associated with direct gloving to determine whether it may be considered compliant according to facility policies.
Inappropriate glove use, during tasks when there is no risk of exposure to infectious matter, or failures to change gloves at appropriate moments during care, has been associated with environmental contamination. Reference Baloh, Thom and Perencevich135,Reference Loveday, Lynam, Singleton and Wilson137 In an observational study, the patient care items most frequently touched by soiled gloves included disinfectant wipes or packaging of patient-care items, patient skin, patient clothing, and durable medical equipment. Reference Burdsall, Gardner and Cox138
In a study of outpatient wound-care providers, hand contamination with a pathogen following doffing of gloves was documented in 10 (19.6%) of 51 encounters. Reference Bingham, Abell and Kienast139 Simulations of doffing using fluorescent gel indicated that the fingertips and wrists were the areas of the hands most likely to be contaminated. Hand contamination was reduced when doffing was modified to include removal of the first glove without touching the hand, followed by inserting the fingers into the dorsal side of the remaining gloved hand to slide the glove off the hand. Reference Alhmidi, Gonzalez-Orta and Cadnum140
Double gloving has been proposed to further reduce hand contamination. In a study examining double gloving using a nonenveloped viral surrogate for Ebola, the inner gloves of 8 of 15 participants were contaminated. One participant who did not have inner glove contamination had hand contamination. These researchers concluded that random contamination events can occur even when double gloving is used, and they emphasized the importance of hand hygiene after doffing. Reference Casanova, Teal and Sickbert-Bennett141
Incorporation of disinfection of gloves with ABHS during task saturated clinical care has also been investigated to reduce hand contamination associated with glove use. These studies were performed independent of glove manufacturers. In one study, disinfection of gloves during care episodes led to increased adherence to hand hygiene. Reference Fehling, Hasenkamp and Unkel142 Exposure to ABHS did not impact tensile strength of nitrile gloves; however, risk of perforation increased when gloves were worn continuously for >15 minutes following wound dressing changes and following patient or resident bathing activities. Reference Hubner, Goerdt and Mannerow143 Researchers also examined disinfection of gloves using bleach wipes prior to doffing; this reduced hand contamination significantly but generated concerns about respiratory irritation associated with use of bleach wipes. Reference Tomas, Sunkesula, Kundrapu, Wilson and Donskey144 Disinfection of gloves prior to doffing is included in CDC guidance on PPE use in response to certain high-consequence pathogens. 145
Wearing gloves for long periods during a work shift increases the risk of occupational irritant or allergic dermatitis. When evaluating allergic dermatitis, it is important to consider ingredients used in the manufacture of gloves, such as rubber accelerators that are used in the manufacture of nitrile gloves. Reference Rundle, Presley and Militello60 Given the risk and benefits associated with glove use, a balanced approach is needed. HCP should be instructed in appropriate use of gloves, facility expectations related to hand hygiene prior to donning gloves, when to change gloves during care, and methods of doffing to reduce hand contamination. Ongoing observations of glove use, donning and doffing as indicated, with immediate performance of hand hygiene following doffing, should be conducted when monitoring adherence to hand hygiene. Fluorescent gel applied to gloves prior to doffing can be a useful tool to educate personnel about hand contamination during doffing. Reference Alhmidi, Gonzalez-Orta and Cadnum140
Presurgical hand antisepsis
The purpose of a surgical hand scrub or rub is to reduce transient and resident organisms on the hands for the duration of the operative procedure. The persistent activity of the surgical hand rubs or scrubs is a key feature of these antiseptics. 66 Waterless surgical hand rubs provide bacterial reductions that are no different than those provided by surgical hand scrubs and are less damaging to the skin. Reference Forer, Block and Frenkel146–Reference Howard, Jowett, Faoagali and McKenzie148 Previous research has shown that scrubbing with a brush may damage skin and increase bacterial shedding from the hands. 11,Reference Parlak, Iyigun, Albay and Bedir149
In a quasi-experimental study using direct overt observation to ensure full compliance with the WHO surgical hand scrub technique, alcohol-based hand scrub improved quality and reduced the duration of the preparation with no significant change in surgical-site infection rates. Reference Gaspar, Menegueti and Lopes150 When comparing a surgical hand scrub formulated with chlorhexidine, waterless surgical hand rub, and povidone iodine, both the CHG and waterless surgical hand scrub had greater reductions of colony-forming units on the hands than povidone iodine. These researchers concluded that preference, compliance, and cost are key to selection of products for presurgical hand antisepsis. Reference Ho, Wang, Loh and Tam147
Scrubbed personnel should pay attention to the amount of waterless product dispensed and increase amounts if needed. Manufacturers often recommend 4–6 mL alcohol-based surgical hand scrub, but individuals with larger hands and forearms may need to use higher volumes. Reference Kampf and Ostermeyer151 The volume used should keep the skin wet for the duration of the surgical hand rub recommended by the manufacturer.
The CDC has recommended against the wear of artificial fingernails or extenders in high-risk areas and makes no statements about jewelry. Cochrane reviewers were able to identify only 1 study comparing wear of freshly applied fingernail polish, old or chipped fingernail polish, and natural fingernails and no random control trials evaluated jewelry. This finding may indicate that prohibitions against wearing of fingernail polish or jewelry by personnel scrubbed for surgical procedures is an accepted practice, and studies involving randomization may pose ethical concerns. Reference Arrowsmith and Taylor152 Because scrubbed personnel are actively interacting with the sterile field, we recommend that fingernails be maintained without polish.
Educational interventions may improve compliance with surgical hand scrubs and surgical hand-rub performance. Reference Gaspar, Menegueti and Lopes150,Reference Abdollahi, Tabrizi, Jodati, Safaie, Moradi-Joo and Daemi153 Structured methods for scrubbing may result in improved technique. Direct overt observation can be used to evaluate technique and to correct lapses during surgical scrubbing. This intervention includes observing for sufficient coverage of arms and adequate time spent performing the scrub. Reference Abdollahi, Tabrizi, Jodati, Safaie, Moradi-Joo and Daemi153 Overt or covert observation may be used to assess ergonomic adjustments needed such as ensuring access to products or placing timers in view of the scrub sink. Fluorescent indicators have been valuable for instructing personnel in proper scrub technique. Reference Gaspar, Menegueti and Lopes150
Section 4. Recommended strategies to improve hand hygiene
Recommendations are categorized as either (1) essential practices that should be adopted by all acute-care hospitals or (2) additional approaches that can be considered in locations and/or populations within hospitals when they are experiencing an outbreak or when HAIs are not controlled despite full implementation of essential practices. Essential practices include recommendations in which the potential to prevent HAIs outweighs the potential for undesirable effects. Additional approaches include recommendations in which the intervention is likely to reduce risk of HAIs, but concern remains regarding the risks for undesirable outcomes, recommendations for which the quality of evidence is low, recommendations in which cost-to-benefit ratio may be high, or recommendations in which evidence supports the impact of the intervention in select settings (eg, during outbreaks) or for select patient populations. Hospitals can prioritize their efforts by initially focusing on implementation of the prevention strategies listed as essential practices. If surveillance or other risk assessments suggest ongoing opportunities for improvement, hospitals should consider adopting some or all of the prevention approaches listed as additional approaches. These can be implemented in specific locations or patient populations or can be implemented hospital-wide, depending on outcome data, risk assessment, and/or local requirements. Each infection prevention recommendation is accompanied by a quality-of-evidence grade (Table 2).
Essential practices for preventing HAIs through hand hygiene
-
1. Promote the maintenance of healthy hand skin and fingernails. 10,Reference Behroozy and Keegel57,Reference Campion58,Reference van der Meer, Boot and van der Gulden154 (Quality of evidence: HIGH)
-
a. Promote the preferential use of ABHS in most clinical situations. 10,Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64 (Quality of evidence: HIGH)
-
b. Perform hand hygiene as indicated by the CDC or the WHO Five Moments (Table 3). (Quality of evidence: HIGH)
-
c. Include fingernail care in facility-specific polices related to hand hygiene:
-
i. HCP should maintain short, natural fingernails.
-
ii. Fingernails should not extend past the fingertip.
-
iii. HCP who provide direct or indirect care in high-risk areas (eg, ICU, perioperative) should not wear artificial fingernail extenders.
-
iv. Prohibitions against fingernail polish (standard or gel shellac) are at the discretion of the infection prevention program, except among scrubbed individuals who interact with the sterile field during surgical procedures; these individuals should not wear fingernail polish or gel shellac.
-
-
d. Include measures for primary and secondary prevention of dermatitis.
-
e. Provide HCP with readily accessible, facility-approved hand moisturizers. Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64
-
f. Engage all HCP in primary prevention of occupational irritant and allergic contact dermatitis. Reference Clemmensen, Randboll, Ryborg, Ebbehoj and Agner62–Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64,Reference van der Meer, Boot and van der Gulden154,Reference van der Meer, Boot, van der Gulden, Jungbauer, Coenraads and Anema155 (Quality of evidence: HIGH)
-
i. Primary prevention of HCP dermatitis should include HCP education about the following:
-
a) Strategies to maintain healthy hand skin
-
b) Handwashing techniques to promote healthy hand skin, such as avoiding hot water and patting rather than rubbing hands dry
-
c) When and how to use gloves, change gloves, take periodic breaks to allow hands to dry, and routinely apply facility-approved moisturizers Reference Clemmensen, Randboll, Ryborg, Ebbehoj and Agner62
-
d) The potential for allergic reactions to components in ABHS formulations, antiseptics (eg, CHG), glove material, or products used during these products’ manufacture (eg, accelerants) Reference Rundle, Presley and Militello60,Reference Barnes, Morgan, Pineles and Harris158
-
-
ii. Provide facility-approved hand moisturizer that is compatible with antiseptics and gloves Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64
-
iii. Evaluate new products for the absence of potential allergenic surfactants, preservatives, fragrances, or dyes Reference Rundle, Presley and Militello60
-
iv. Workplace self-screening for dermatitis Reference Nichol, Copes, Spielmann, Kersey, Eriksson and Holness159,Reference Nichol, Copes, Kersey, Eriksson and Holness160
-
v. Refer HCP to the occupational health department for assistance in cases of hand eczema or dermatitis
-
-
g. Provide cotton glove liners for HCP with hand irritation and educate these HCP on their use (ie, following instructions for use, laundering, and/or discarding). Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64 (Quality of evidence: MODERATE)
-
-
2. Select appropriate products. (Quality of evidence: HIGH)
-
a. For routine hand hygiene, choose liquid, gel, or foam ABHS with at least 60 % alcohol. Reference Bruhwasser, Hinterberger and Mutschlechner8,10,65,Reference Siddharta, Pfaender and Vielle76,Reference Edmonds, Macinga and Mays-Suko79,Reference Ory, Zingg, de Kraker, Soule and Pittet94 (Quality of evidence: HIGH)
-
b. Involve HCP in the selection of products. Reference Ho, Wang, Loh and Tam147 (Quality of evidence: HIGH)
-
c. Obtain and consider manufacturers’ product-specific data if seeking ABHS with ingredients that may enhance efficacy against organisms anticipated to be less susceptible to biocides. Reference Kampf78,Reference Edmonds, Macinga and Mays-Suko79 (Quality of evidence: MODERATE)
-
d. Confirm that the volume of ABHS dispensed is consistent with the volume shown to be efficacious. Reference Macinga, Edmonds, Campbell, Shumaker and Arbogast89,Reference Bellissimo-Rodrigues, Soule, Gayet-Ageron, Martin and Pittet95,Reference Park, Kim and Lim98 (Quality of evidence: HIGH)
-
e. Educate HCP about the appropriate volume of ABHS and the time required to be effective. Reference Bellissimo-Rodrigues, Soule, Gayet-Ageron, Martin and Pittet95 (Quality of evidence: HIGH)
-
i. The volume of hand sanitizer should be sufficient to cover all surfaces of the hands and may require >1 dispenser actuation for large hands. Reference Bellissimo-Rodrigues, Soule, Gayet-Ageron, Martin and Pittet95 (Quality of evidence: HIGH)
-
ii. When sanitizing, HCP should rub hands for a minimum of 15 seconds. When handwashing, HCP should scrub for a minimum of 15 seconds. 53,Reference Harnoss, Dancer and Kaden96,Reference Kramer, Pittet and Klasinc161,Reference Stahmeyer, Lutze, von Lengerke, Chaberny and Krauth162 (Quality of evidence: HIGH)
-
iii. Facilities should consider fluorescent indicators for use when training HCP in the application of ABHS and handwashing.
-
-
f. Provide facility-approved hand moisturizer that is compatible with antiseptics and gloves. Reference van der Meer, van der Gulden, van Dongen, Boot and Anema64 (Quality of evidence: HIGH)
-
g. For surgical antisepsis, use an FDA-approved surgical hand scrub or waterless surgical hand rub. (Quality of evidence: HIGH)
-
i. Complete surgical hand antisepsis by performing a surgical hand rub or surgical hand scrub. (Quality of evidence: HIGH)
-
ii. Scrub brushes should be avoided because they damage skin. (Quality of evidence: HIGH)
-
-
-
3. Ensure the accessibility of hand hygiene supplies. (Quality of evidence: HIGH)
-
a. Ensure that ABHS dispensers are unambiguous, visible, and accessible within the workflow of HCP. Reference Cure and Van Enk105–Reference Smolkin, Roth-Ahronson and Kranzler111 (Quality of evidence: HIGH)
-
i. Use a systematic method (eg, workflow evaluation, event counters) to determine optimal placement of ABHS dispensers. (Quality of evidence: HIGH)
-
-
b. In private rooms, consider 2 ABHS dispensers per private room the minimum threshold for adequate numbers of dispensers: 1 dispenser in the hallway, and 1 dispenser in the patient room. Reference Chan, Homa and Kirkland102 (Quality of evidence: HIGH)
-
c. In semiprivate rooms, suites, bays, and other multipatient bed configurations, consider 1 dispenser per 2 beds as the minimum threshold for adequate numbers of dispensers. Place ABHS dispensers in the workflow of HCP. Reference Diefenbacher, Fliss, Tatzel, Wenk and Keller48 (Quality of evidence: LOW)
-
d. Ensure that the placement of hand hygiene supplies (eg, individual pocket-sized dispensers, bed mounted ABHS dispenser, single use pump bottles) is easily accessible for HCP in all areas where patients receive care. Reference Carter, Wyer and Giglio103,Reference Salmon and McLaws104 (Quality of evidence: HIGH)
-
e. Evaluate for the risk of intentional consumption. Utilize dispensers that mitigate this risk, such as wall-mounted dispensers that allow limited numbers of activations within short periods (eg, 5 seconds). (Quality of evidence: LOW)
-
i. If individual pocket-sized dispensers are used when caring for individuals at risk for intentional consumption, they must always remain in the control of the HCP.
-
-
f. Have surgical hand rub and scrub available in perioperative areas. (Quality of evidence: HIGH)
-
g. Consider providing ABHS hand rubs or handwash with FDA-approved antiseptics for use in procedural areas and prior to high-risk bedside procedures (eg, central-line insertion). (Quality of evidence: LOW)
-
-
4. Ensure appropriate glove use to reduce hand and environmental contamination. Reference King, López-García and Atedoghu130–Reference Landelle, Verachten, Legrand, Girou, Barbut and Brun-Buisson132,Reference Burdsall, Gardner and Cox138 (Quality of evidence: HIGH)
-
a. Use gloves for all contact with the patient and environment as indicated by standard and contact precautions during care of individuals with organisms confirmed to be less susceptible to biocides (eg, C. difficile or norovirus). 10
-
i. HCP caring for preterm neonate with central lines should perform hand hygiene before donning nonsterile gloves prior to patient and vascular device contact. Reference Kaufman, Blackman, Conaway and Sinkin133 (Quality of evidence: HIGH)
-
-
b. Educate HCP about the potential for self-contamination and environmental contamination when gloves are worn. (Quality of evidence: HIGH)
-
i. Whenever hand hygiene is indicated during episodes of care, HCP should doff gloves and perform hand hygiene.
-
-
c. Clean hands immediately following glove removal. If handwashing is indicated and sinks are not immediately available, use ABHS and then wash hands as soon as possible.
-
d. Educate and confirm the ability of HCP to doff gloves in a manner that avoids contamination. (Quality of evidence: HIGH)
-
i. Consider using fluorescent indicators applied to gloves during demonstrations of doffing to help HCP visualize how contamination may occur.
-
-
-
5. Take steps to reduce environmental contamination associated with sinks and sink drains. Reference Cloutman-Green, Kalaycioglu and Wojani114,Reference Balm, Salmon and Jureen116–Reference Roux, Aubier, Cochard, Quentin and van der Mee-Marquet123 (Quality of Evidence: HIGH)
-
a. Ensure that handwashing sinks are constructed according to local administrative codes.
-
b. Include handwashing sinks in water infection control risk assessments for healthcare settings.
-
c. If possible, dedicate sinks to handwashing.
-
d. Educate HCP to refrain from disposing substances that promote growth of biofilms (eg, intravenous solutions, medications, liquid food, or human waste) in handwashing sinks.
-
e. Use an EPA-registered hospital disinfectant to clean sink bowls and faucets daily.
-
f. Do not keep medications or patient care supplies on countertops or mobile surfaces that are within 1m (3 feet) of sinks.
-
i. Install splash guards if countertops must be used to store supplies.
-
-
g. Provide disposable or single-use towels to dry hands. Do not use hot air dryers in patient care areas.
-
h. Consult with state or local public health officials when investigating confirmed or suspected outbreaks of healthcare-associated infections due to waterborne pathogens of premise plumbing.
-
-
6. Monitor adherence to hand hygiene. (Quality of evidence: HIGH)
-
a. Use multiple methods to measure adherence to hand hygiene.
-
b. Consider advantages and limitations of each type of monitoring.
-
i. Direct observation
-
a) Direct overt observation Reference Wu, Lee and Chen20,Reference Abdollahi, Tabrizi, Jodati, Safaie, Moradi-Joo and Daemi153
-
1) To evaluate and improve HCP technique and adherence to facility-specific policies
-
2) To prevent lapses during high-risk procedures such as insertion of invasive devices.
-
-
b) Direct covert observation Reference Livorsi, Goedken and Sauder14,Reference Baek, Kim, Kim, Cho, Hong and Kim16,Reference El-Saed, Noushad and Tannous17,Reference Srigley, Furness, Baker and Gardam19,Reference Yin, Reisinger and Vander Weg25,Reference Chang, Reisinger and Jesson47
-
1) To monitor rates of adherence
-
2) To elucidate contextual barriers and facilitators to hand hygiene
-
3) To provide corrective feedback to individuals.
-
-
-
-
c. Use a systematic approach to determine where and when observations should occur. Reference Fries, Segre, Thomas, Herman, Ellingson and Polgreen23,Reference Sunkesula, Meranda and Kundrapu24
-
i. Provide training for individuals who will collect observations. Ensure observers are prepared to address nonadherence.
-
ii. Limit observation periods to no more than 15 minutes.
-
iii. Collect enough observations to detect statistically significant changes in practice.
-
-
d. Use an AHHMS to monitor trends in adherence on all shifts and days of the week. Reference Doll, Masroor and Cooper26,Reference Helder, Weggelaar and Waarsenburg163
-
i. Collaborate with HCP in the implementation of an AHHMS and empower them to identify ways to improve the system (eg, who to notify when real-time reminders are not accurate or when maintenance is needed). Reference Benudis, Stone and Sait33,Reference Edmisten, Hall and Kernizan34
-
-
e. Use patient-as-observer methods in areas with limited resources, such as outpatient departments. Reference Le-Abuyen, Ng and Kim38
-
f. Use product volume measurement for large-scale planning and benchmarking.
-
i. Audit the accessibility and functionality of hand hygiene equipment and supplies to ensure hand hygiene is supported by the physical environment of care. Reference Cawthorne and Cooke22
-
-
-
7. Provide timely and meaningful feedback to enhance a culture of safety. Reference Ivers, Jamtvedt and Flottorp50–Reference Sánchez-Carrillo, Rodríguez-López and Galarza-Delgado52 (Quality of evidence: MODERATE)
-
a. Provide feedback in multiple formats (eg, verbal, written) and on multiple occasions (ie, real-time, weekly). Reference Ivers, Jamtvedt and Flottorp50
-
b. Consider debriefing unit managers as soon as possible after each direct covert observation session. This can be done in a manner that preserves the observer’s confidentiality.
-
c. Provide meaningful data with clear targets linked to actions to improve adherence. Reference Ivers, Jamtvedt and Flottorp50
-
i. Meaningful data may include unit or role-based adherence data rather than overall performance. 164
-
ii. Real-time displays of hand hygiene adherence may provide incentive for improvement on a shift-by-shift basis.
-
-
Additional approaches to prevent HAIs through hand hygiene during outbreaks
-
1. Consider educating HCP using a structured approach (eg, WHO steps) for handwashing or hand sanitizing. Evaluate HCP adherence to technique. (Quality of evidence: LOW)
-
2. For waterborne pathogens in the plumbing of the facility, consider disinfection of sink drains using an EPA-registered disinfectant with claims against biofilms. Consult with state or local public health for assistance in determining appropriate protocols for use and other actions needed to ensure safe supply. (Quality of evidence: LOW)
-
3. For C. difficile and norovirus, in addition to contact precautions, encourage hand washing with soap and water after the care of patients with known or suspected infections. (Quality of evidence: LOW)
Approaches that should not be considered part of routine hand hygiene
-
1. Do not supply individual pocket-sized ABHS dispensers in lieu of minimum thresholds for accessible wall-mounted dispensers.
-
2. Do not refill or “top-off” soap dispensers, moisturizer dispensers, or ABHS dispensers intended for single use. 127
-
3. Do not use antimicrobial soaps formulated with triclosan as an active ingredient. 67
-
4. Do not routinely double-glove except when specifically recommended for certain job roles or in response to certain high-consequence pathogens. Reference Casanova, Teal and Sickbert-Bennett141
-
a. Certain scrubbed surgical team members must wear double gloves because of the risk for glove perforations that may contaminate sterility or expose the HCP to infectious materials.
-
b. Anesthesia personnel may wear double gloves during airway management.
-
c. Personnel compounding medications according to the US Pharmacopeia 797 may be required to wear double gloves.
-
-
5. Do not routinely disinfect gloves during care except when specifically recommended in response to certain high-consequence pathogens.
-
6. Do not remove access to ABHS for HCP responding to organisms that are anticipated to be less susceptible to biocides (eg, C. difficile, norovirus). 11
-
7. Do not attempt to remediate potential biofilms in sink drains with disinfectants lacking EPA registration for this use.
Unresolved issues
-
1. HCP use of alcohol-impregnated hand wipes is unresolved due to the lack of noninferiority data. Reference Ory, Zingg, de Kraker, Soule and Pittet94
Section 5. Performance measures
Internal reporting
Hand hygiene adherence measurement is not standardized in the United States and will depend on the methods used by the facility and its goal for monitoring. It is intended to support internal quality improvement through measurement, feedback, and longitudinal assessment of interventions at individual facilities or clusters of facilities in the same health system. A list of performance measures for internal reporting is provided in Table 6.
Table 6. Metrics for Reporting Adherence to Hand Hygiene
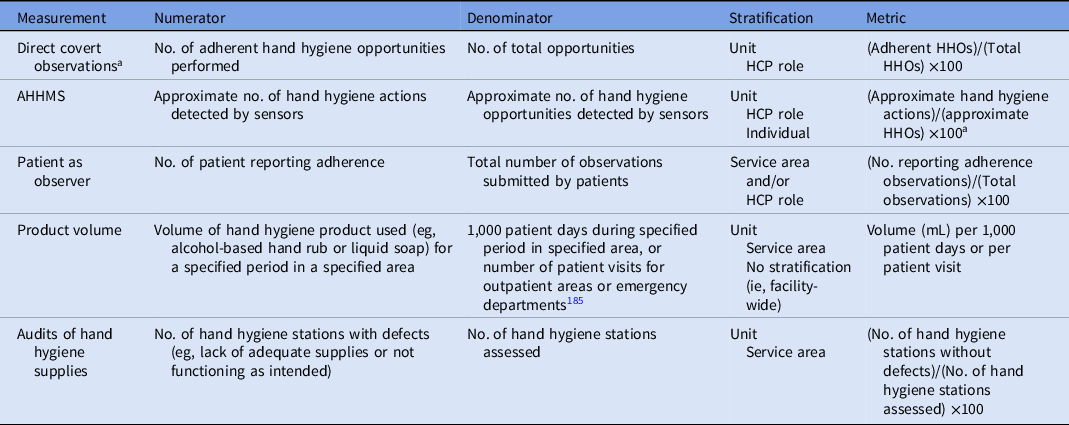
a Direct overt observation should not be used to calculate adherence.
External reporting
There continues to be no requirement in the United States for external reporting of adherence to hand hygiene. Because the credibility of observational methods has yet to be established, any publicly reported hand hygiene metric will suffer from distrust of the data due to misaligned incentives. Reference Ellingson, Haas and Aiello1 When on site, the Centers for Medicaid and Medicare and accrediting organizations [eg, The Joint Commission or Det Norske Veritas (DNV)] evaluate several aspects of hand hygiene programs, including accessibility of supplies, initiatives to improve HCP adherence, measurement methods, and adherence to state-specific administrative code.
Section 6. Implementation strategies
Leadership at all levels plays a role in hand hygiene improvement, and accountability begins with the chief executive officer and other senior leaders who provide the imperative for HAI prevention, thereby making it an organizational priority. Senior leadership is accountable for providing adequate resources, including necessary personnel, equipment, and assistance when escalating situations of continued nonadherence. Directing interventions toward unit-based managers and department leaders to improve team functioning and supporting their personnel have been reported to be more effective in improving hand hygiene adherence than interventions aimed toward individuals. Reference Huis, Schoonhoven, Grol, Donders, Hulscher and van Achterberg165 Hand hygiene champions, whether informal leaders or formally appointed HCP, have been associated with improved hand hygiene when they provide effective, collegial communication to frontline HCP. Reference Rosenbluth, Garritson and Green166
In general, studies examining the association between hand hygiene improvement programs and increases in hand hygiene adherence (and/or decreases in healthcare-associated infections) do not meet the quality standards required of meta-analytic reviews. A Cochrane review published in 2017 included 26 studies of various combinations of interventions, but strategies for hand hygiene improvement were categorized as having low levels of certainty. Reference Gould, Moralejo, Drey, Chudleigh and Taljaard170 Several elements of the implementation strategies are unchanged from those provided in 2014. Reference Ellingson, Haas and Aiello1
Engage
-
1. Develop a multidisciplinary team that includes representatives from administrative leadership as well as unit and department managers and unit-level and department-level champions.
-
a. Align hand hygiene goals with the organizational mission and vision for high-quality patient care.
-
b. Assure that institutional leadership is aware and supportive of hand hygiene improvement strategies and supports these efforts with adequate resources.
-
c. When implementing improvement programs, secure the active commitment of unit and department-based leadership. Set targets in collaboration with leaders and teams. Reference Diefenbacher, Fliss, Tatzel, Wenk and Keller48,Reference Huis, Holleman, van Achterberg, Grol, Schoonhoven and Hulscher171
-
1. Ensure that unit and department managers hold the HCP they supervise accountable for hand hygiene performance. Reference Huis, Holleman, van Achterberg, Grol, Schoonhoven and Hulscher171
-
-
-
2. Utilize peer networking to encourage persistent salience of hand hygiene. Reference Ellingson, Haas and Aiello1
-
a. Consider rewards or recognition for wards modeling good hand hygiene behaviors or improvement. Qualitative studies suggest that role modeling, particularly that of physicians, is important yet underappreciated. Reference Rosenbluth, Garritson and Green166,Reference Pedersen, Elgin and Peace172
-
-
3. Identify barriers and facilitators to hand hygiene adherence specific to the unit or institution. Facilitators of adherence may be as simple as having a place to set items prior to entering the patient environment. This information is then used to create interventions specific to their needs. Reference Chassin, Mayer and Nether173,Reference Kurtz174
-
4. Consider enthusiastically inviting patients to take an active role in reminding HCP to perform hand hygiene
-
a. Ensure HCP respond to patient requests for them to perform hand hygiene in a positive manner. Reference Grota, Eng and Jenkins175 Consider providing a brief script (ie, “Thank you for reminding me.”)
-
b. Utilize patient education materials on the CDC website. 176
-
Educate
-
1. Educate HCP and assure knowledge and skill on the following items:
-
a. The importance of hand hygiene in reducing the risk for HAI
-
b. HHOs using WHO Five Moments or CDC indications
-
c. Fingernail and hand condition, primary prevention of dermatitis
-
d. Facility-specific policies regarding jewelry
-
e. Delineation of the patient zone, particularly when patients are housed in bays or crowded areas
-
f. Technique, ensuring coverage of all hand surfaces, duration of hand rubbing or washing
-
g. Use of hand-care products that are compatible with hand hygiene products specific to the area in which HCP work
-
h. Use of gloves in a manner that reduces hand or environmental contamination.
-
-
2. Use interactive methods to educate HCP about technique for hand sanitizing, handwashing, and doffing of gloves.
-
3. Use short, frequent educational interventions to continually build HCP knowledge and practice of hand hygiene.
-
a. Assess HCP knowledge of hand hygiene with written tests or quizzes.
-
b. Assess HCP skill in hand hygiene and use of gloves by return demonstration.
-
-
4. Use principles of adult education to encourage participation and ongoing learning.
Execute
-
1. Provide access to ABHS within the workflow of HCP.
-
2. Implement a multimodal (ie, bundled) hand hygiene improvement program. Accessibility and visibility of dispensers and supplies may be the most important bundle element. Reference Cure and Van Enk105,Reference Scheithauer, Hafner, Schroder, Nowicki and Lemmen108,Reference Cai, Tyne, Spreckelmeyer and Williams177 A culture of safety that penetrates to the individual level (ie, psychological safety) has also been associated with improved hand hygiene. Reference Jimmieson, Tucker and White178 Real-time verbal or electronic reminders to perform hand hygiene are likely more effective than signage. Reference Birnbach, Rosen, Fitzpatrick, Everett-Thomas and Arheart179 Interventions must be ongoing to maintain behavior change and improved adherence. Reference Kwok, Juergens and McLaws180
-
3. Focus on targeted behavior change. Posters, if used, should be motivational in nature rather than simply conveying information. Emphasize the protective nature of hand hygiene and altruism. Reference Mackert, Lazard and Champlin181
Evaluate
-
1. Measure hand hygiene adherence performance. A combination of approaches may be most appropriate (see Section 2).
-
2. Measurement may need to be adjusted for facility-specific needs. Use or build upon existing tools:
-
a. WHO observation forms are available online. 182
-
b. A variety of other forms are available for free in The Joint Commission’s hand hygiene monograph. 164
-
c. The Joint Commission Center for Transforming Healthcare’s Targeted Solutions Tool for Hand Hygiene are available free for organizations accredited by The Joint Commission. 183
-
d. Several iOS and Android applications, including the iScrub application, are available to assist with direct observation. 184
-
-
3. Provide meaningful feedback on hand hygiene performance with clear targets connected with an action plan in place for improving adherence.
-
a. Feedback of hand hygiene adherence rates has long been recognized as an important component of multimodal hand hygiene improvement program, although the independent impact of feedback apart from other bundled hand hygiene interventions is not known.
-
b. Feedback may be most effective when provided more than once, when both verbal and written feedback are provided, and when a superior or colleague is responsible for the audit and feedback. Reference Ivers, Jamtvedt and Flottorp50
-
c. Providing overall hand hygiene adherence rates for a facility may not be as effective as unit based or role-based reports at identifying problem areas and planning focused training efforts.
-
d. Hand hygiene data may be displayed on dashboards that provide the most recent or cumulative hand hygiene adherence rates compared with a target rate. Statistical process control charts can be used to show data trends over time and whether changes in rates are due to specific interventions or normal variation. Some automated monitoring systems can give real-time displays of hand hygiene adherence on the unit, providing some incentive for improvement on a shift-by-shift basis.
-
e. Use feedback to engage HCP in identifying problems at individual hospital or unit level and use data to tailor ongoing interventions.
-
f. If individually identified hand hygiene adherence rates are used, consider providing feedback privately versus in a public staff setting.
-
g. Some facilities report hand hygiene adherence data in conjunction with hospital-associated infection rates. Although the association between hand hygiene and HAI reductions has been reported in the literature, the association may not be evident in individual unit or facility data due to confounding factors (eg, environmental cleanliness and small sample sizes).
-
h. Use monitoring data to inform action plans at the most specific level possible (administrative area, service line, unit, or even individual) and follow through on improving these process measures as a step towards improving hand hygiene overall.
-
Hand hygiene programs should strive to create a culture of safety in which all HCP collaborate to protect patients or residents. Interprofessional dialogue and safe spaces for learning about hand hygiene provide motivation and engagement of HCP. Reference Erichsen Andersson and Frodin167 Strategies for implementation of multimodal hand hygiene improvement programs, including system and/or infrastructure change (eg, availability of alcohol-based hand rubs), education, evaluation and feedback, reminders (eg, posters), and institutional safety climate (eg, administrative support), have been endorsed and detailed by the WHO in the 2009 publication entitled A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. 11,168 Resources are available on the CDC Hand Hygiene in Healthcare Settings webpage and from other organizations such as The Institute for Healthcare Improvement’s “How to” guide 169 and The Joint Commission Center for Transforming Healthcare Targeted Solutions Tool (or TST) for Hand Hygiene. Reference Huis, Holleman, van Achterberg, Grol, Schoonhoven and Hulscher171
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
A.A. reports payment from the Analysis Group for serving as an expert consultant on litigation related to the prevention of COVID-19. K.H. reports consulting for Medillum and Nozin, a grant from the NC Department of Public Health, and providing a lecture for PDI. R.O. reports consulting for Specified Technologies and Northshore University Health System, providing expert testimony for Saxton & Stump, providing a lecture for Teleflex Medical Advisory Board, subject-matter expertise for APIC for AHA HRET, and peer review of a manuscript for Joint Commission Resources. All other authors report no conflicts of interest related to this article.









