Early reports from China indicate that severe acute respiratory coronavirus virus 2 (SARS-CoV-2) RNA may persist in the respiratory tracts of patients with coronavirus disease 2019 (COVID-19) for several weeks after symptom onset.Reference Zhou, Yu and Du1–Reference Xing, Mo, Xiao, Zhao, Zhang and Wang3 However, the duration of SARS-CoV-2 RNA shedding has not been systematically studied in a large cohort of patients.
Methods
To estimate the duration of SARS-CoV-2 RNA shedding, we conducted a multisite study among patients who had nasopharyngeal specimens tested for SARS-CoV-2 RNA via real-time polymerase chain reaction (PCR) assay at Providence St Joseph Health (a 51-hospital healthcare organization based in Renton, Washington), University of Chicago Medicine in Chicago, Illinois, and NorthShore University HealthSystem (a 5-hospital healthcare system based in Evanston, Illinois). All patients with a positive SARS-CoV-2 PCR test between January 22, 2020, and April 23, 2020 who had at least 1 subsequent SARS-CoV-2 PCR test were included in the study. SARS-CoV-2 PCR tests were ordered at the discretion of medical providers at each institution. We calculated the percentage of patients with a persistent positive SARS-CoV-2 PCR test result up to 25 days after the first positive test. This study was approved by the institutional review board of each institution.
Results
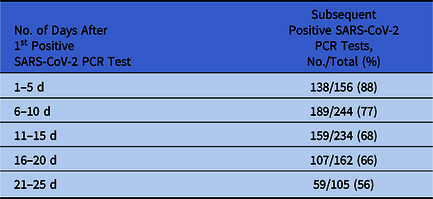
During the study period, 76,040 SARS-CoV-2 PCR tests were performed among 70,406 unique patients. The mean age of all patients tested was 48.3 years. Of these patients, 10,584 (15%) tested positive for SARS-CoV-2. Of these 10,584 patients, 555 (5%) with an initial positive test for SARS-CoV-2 RNA underwent at least 1 subsequent SARS-CoV-2 PCR test within 25 days of the first test. The mean age of patients who tested positive and had a subsequent test was 61.7 years. Among 156 patients with a subsequent test 1–5 days after their initial positive test, 138 (88%) continued to have a positive test (Table 1). Among 105 patients with a subsequent test 21–25 days after their initial positive test, 59 (56%) continued to have a positive test.
Table 1. Duration of SARS-CoV-2 RNA Detection

Note. PCR, polymerase chain reaction assay.
Discussion
In this multicenter US study, we found that SARS-CoV-2 RNA shedding persists for >3 weeks in most patients with COVID-19. This finding has important implications for infection prevention in both inpatient and outpatient settings. The Centers for Disease Control and Prevention recommends 2 possible strategies for determining when isolation precautions can be discontinued for symptomatic patients with COVID-19: a symptom-based strategy and a test-based strategy.4 In the symptom-based strategy, isolation precautions can be discontinued 3 days after patient recovery and 10 days after symptom onset, whichever is longer. In the test-based strategy, isolation precautions can be discontinued after improvement in symptoms and at least 2 negative SARS-CoV-2 PCR tests collected at least 24 hours apart.4 Our findings that SARS-CoV-2 PCR tests remain positive for >3 weeks in most patients suggest that patients following the test-based strategy may remain on precautions for prolonged periods.
Our results are consistent with smaller studies that have also found prolonged duration of SARS-CoV-2 RNA positivity among patients with COVID-19.Reference Zhou, Yu and Du1,Reference He, Lau and Wu2,Reference Xiao, Tong and Zhang5,6 He et alReference He, Lau and Wu2 examined the dynamics of viral shedding among 94 patients with COVID-19 and found that the SARS-CoV-2 tended to decrease below the detectable limit ~21 days after symptom onset. Xiao et alReference Xiao, Tong and Zhang5 examined 56 patients with COVID-19 and found a median time from symptom onset to negative PCR test of 24 days. A positive PCR test does not necessarily correlate with viral transmissibility. Indeed, others have found no viable SARS-CoV-2 virus in culture among patients with prolonged SARS-CoV-2 RNA detection.Reference Wolfel, Corman and Guggemos7–9
Our study has several limitations. It was a retrospective cohort study among patients with COVID-19 who underwent SARS-CoV-2 PCR testing at the discretion of their medical providers. Patients with COVID-19 who are subsequently retested for SARS-CoV-2 are often inpatients being considered for transfer to a nursing home or other long-term care facility. These patients may be older and have more chronic medical conditions than COVID-19 patients in the outpatient setting, so our findings may not be representative of all individuals with COVID-19. We did not collect the clinical characteristics of patients in this analysis. In addition, we were not able to assess SARS-CoV-2 PCR test results in relation to the timing of symptom onset. Patients typically develop symptoms before they undergo their first SARS-CoV-2 PCR test. Therefore, our findings likely underestimate the duration of SARS-CoV-2 RNA shedding.
In conclusion, in a multisite cohort study, we found prolonged duration of SARS-CoV-2 RNA shedding among patients with COVID-19. More research is needed to understand the duration of SARS-CoV-2 transmissibility among patients with COVID-19.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.