Antimicrobial resistance (AMR) is globally recognized as a major public health threat. The World Bank has estimated that the economic losses due to AMR between now and 2050 could be as substantial as those provoked by the 2008–2009 global financial crisis.1 A large proportion of the burden of infections caused by AMR bacteria is due to healthcare-associated infections (HAIs).Reference Cassini, Högberg and Plachouras2 Preventing HAIs and optimizing antimicrobial usage (AMU) are both essential for tackling AMR.3
The European Centre for Disease Prevention and Control (ECDC) promotes and coordinates the standardized measurement of HAIs and AMU in acute-care hospitals across the European Union through repeated point-prevalence surveys (PPSs). The first study was conducted in 2011–2012 in 29 EU countries and Croatia; the results revealed that 32.7% of patients received 1 or more antimicrobial agents on the day of the survey.4 The second PPS was conducted in 2016–2017, and 28 EU/European Economic Area (EEA) countries and Serbia participated. The observed prevalence of AMU was 32.9%.Reference Plachouras, Kärki and Hansen5 Italy participated in both surveys. In the first Italian survey, conducted in 2011, the AMU prevalence was 44.0%, and several issues regarding AMU and AMR were identified, such as the widespread use of carbapenems and high levels of carbapenem-resistant microorganisms.6
We present AMU data from the second Italian PPS, conducted in 2016. Through the assessment of AMU prevalence and the evaluation of prescribed agents and indications, the aim of this study was to improve knowledge regarding AMU in Italy and to identify targets for future interventions to promote a more prudent use of antimicrobials in acute-care settings.
Methods
Protocol and definitions
A standardized protocol was applied in all countries participating in the PPS.7 The Department of Public Health Sciences and Pediatrics of the University of Turin was the Italian national coordinating center for the 2016 survey.
The PPS protocol adopted European case definitions for HAIs (ie, Hospitals in Europe Link for Infection Control through Surveillance, HELICS) as well as US HAI definitions (ie, National Healthcare Safety Network, NHSN).Reference Zarb, Coignard and Griskeviciene8 Antimicrobial groups and agents were classified according to the World Health Organization Collaborating Centre for Drug Statistics Anatomic Therapeutic Chemical index.9 In our study, AMU prevalence was defined as the percentage of patients receiving at least 1 antimicrobial agent on the day of the survey. We used the definition of broad-spectrum and/or last-line agents (BS/LLAs) proposed by the 2017 ECDC, European Food Safety Authority and European Medicines Agency Joint Scientific Opinion.10
Participating hospitals
For the 2016 survey, acute-care hospitals in Italy were invited to participate in the PPS on a voluntary basis. The total number of participating hospitals was 135. According to the ECDC, each region in Italy was required to participate with at least 3 hospitals of different size classes. Due to the heterogeneity in regional participation, a subsampling procedure was performed. From the list of participating hospitals, 56 hospitals were subsampled in accordance with ECDC indications.
Data collection
The Italian PPS was conducted between October and November 2016. Data were collected by trained local hospital staff on 1 day per ward, and data collection in each hospital was completed within 3 weeks. All patients admitted to the ward before 8 a.m. on the day of the survey and still present at the time of the PPS were included.
Because the survey’s aims were the surveillance of diseases and the improvement of healthcare quality and because the program was coordinated by public entities (the University of Turin in 2016, the Regional Health Agency of the region of Emilia-Romagna in 2011, the Italian Centre for Disease Control and Ministry of Health), the written consent of patients was not requested. Patients were provided with an information sheet to notify them of their participation in the survey. Only anonymized data were collected and sent to the national coordinating center. For each region, the approval from at least 1 local health unit’s ethics commission was obtained.
Demographic and clinical data were collected for each patient, including risk factors such as the presence of invasive devices and the severity of underlying medical conditions according to the McCabe score.7 For patients receiving 1 or more antimicrobial on the day of the survey (or in the previous 24 hours in case of surgical prophylaxis) additional information was collected: antimicrobial agent and group, route of administration, dosage, indication for antimicrobial use, anatomical site of infection (in case of treatment indication), and whether the reason for AMU was documented in the patient’s chart. For active HAIs, on the day of the survey, further HAI data were collected, including microbiological test results and susceptibility to selected AMR markers. Structure and process indicators at hospital level in relation to HAI prevention and antimicrobial stewardship were also collected.
Validation study
For the current survey, a validation study was performed in 5 randomly selected hospitals of the representative sample, and 254 patient charts were re-examined. Using validation data, the ECDC provided an AMU prevalence estimate adjusted for misclassification risk.
Statistical analysis
Data were collected and managed using HelicsWin v2.3.4 software (ECDC). Between December 2016 and June 2017, data were transferred to the national coordinating center for quality assessment, assembly, and analysis. Analyses were performed using R version 3.4.0 software (R Foundation for Statistical Computing, Vienna, Austria). Poisson regression was used to compare 2011 and 2016 data. Prevalence ratios (PRs), 95% confidence intervals (CIs), and P values are reported. Binomial testing was used to report 95% CI for prevalence estimates.
Results
In the 2016 national survey, data on 28,157 patients were collected from 135 hospitals. The representative sample included 14,773 patients from 56 hospitals. A national report with the complete results was published in 2018.11 For the current study, data from the 2016 sample were compared with data from the representative sample of the 2011 national survey,6 which included 14,784 patients from 49 hospitals. Demographic and clinical data of patients included in the representative samples of both surveys are shown in Table 1.
Table 1. Demographic Data and Clinical Data of Patients Included in the Representative Samples of the 2011 Survey (n = 14,787) and the 2016 Survey (n = 14,773)
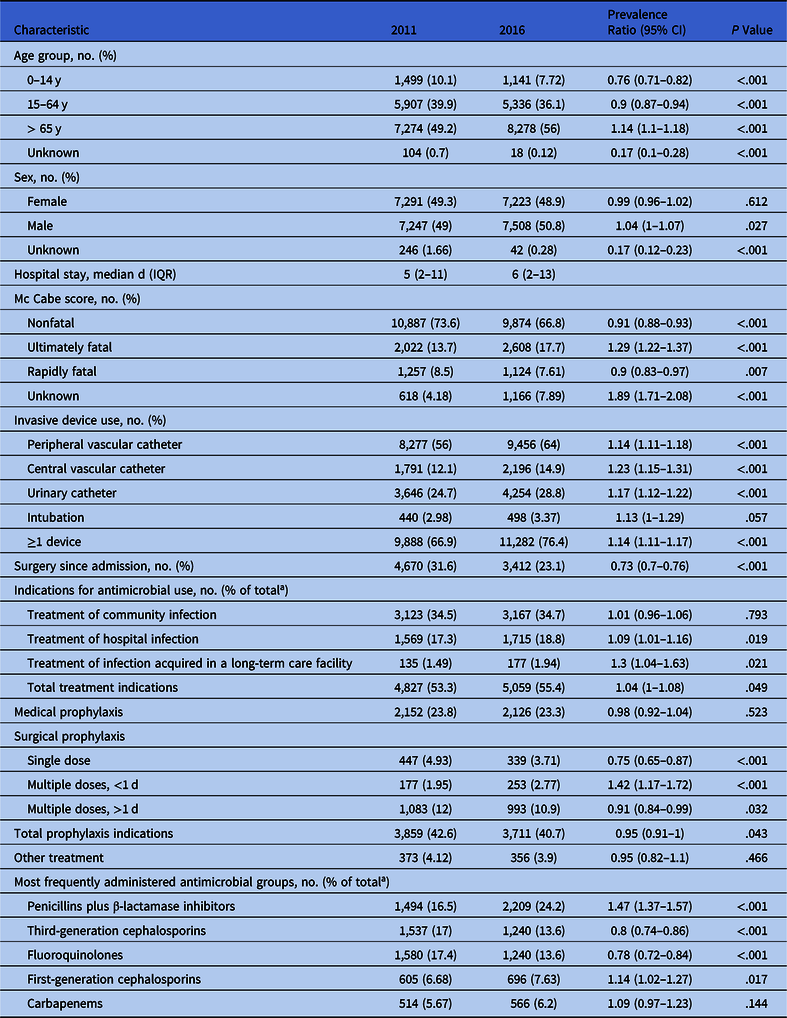
a A total of 9,059 antimicrobial agents were reported in 2011 and 9,126 were reported in 2016.
Prevalence and indications for antimicrobial use
Among all patients included in the 2016 survey, 6,574 received at least 1 antimicrobial agent, and the overall AMU prevalence was 44.5% (95% CI, 43.7–45.3). No significant change in AMU prevalence was observed (P = .535) when the data were compared with the 2011 survey (AMU prevalence, 44.0%; 95% CI, 42.1–45.7). In total, 9,126 antimicrobial prescriptions were recorded (mean per patient, 1.39; 95% CI, 1.34–1.43). The mean AMU rates in 2016 ranged from 6.0% in psychiatry to 64.3% in intensive care departments, and the mean AMU rates were 47.9% for medical specialties, 50.6% for geriatric care, and 51.0% for surgical specialties.
Analyzing interregional variations, we detected significant differences: the prevalence of AMU was 43% in the northern region, 44.7% in the central region, and 50.1% in the southern region of Italy. We also detected a significant gradient from north to south (risk ratio, 1.05; 95% CI, 1.04–1.07; P < .001).
The validation study revealed a sensitivity of 93.9% and a specificity of 88.5% in identifying AMU. The ECDC estimate for the 2016 Italian AMU rate was 39.6% (95% CI, 34.4–45.0).
The most prevalent indication for AMU was the treatment of infections in both surveys (Table 1). The most common infections for which antimicrobials were prescribed in 2016 were pneumonia (29.3%), cystitis or other symptomatic lower urinary tract infections (9.0%), and laboratory-confirmed bacteremia (8.5%).
In 2016, an indication was documented in the patient’s medical records for 6,857 antimicrobials (75.1%). The highest percentages of documented indications were reached in neonatal (86.3%) and rehabilitation (82.8%) specialties, whereas the lowest percentages were found in long-term care (58.7%) and gynecology and obstetrics (62.7%).
Antimicrobial agents
Considering all indications, penicillins plus BLIs were the most common antimicrobial group in 2016; they were used significantly more than in 2011 (Table 1). Piperacillin plus BLI was the most frequently used agent in 2016, accounting for 13.3% of all AMs.
In 2016, 8,346 antimicrobials for systemic use were prescribed. Also, BS/LLAs accounted for 60.8% of antimicrobials for systemic use considering all indications, 69.5% considering treatment indications, 57.5% for medical prophylaxis, and 30.4% for surgical prophylaxis (Fig. 1).

Fig. 1. Proportion of broad-spectrum/last line (BS/LL) antimicrobials10 among antimicrobials for systemic use per indication in the representative sample of the 2016 survey (n = 8,346).
Antimicrobial resistance
In 2016, 1,296 HAIs were identified, and for 53.8% of these, a positive microbiology result was available. Overall, 841 microorganisms were identified in 2011 and 876 were identified in 2016. Table 2 shows the frequency of AMR for selected microorganisms.
Table 2. Antimicrobial (AM) Resistance of Selected Microorganisms Isolated From Infections in the Representative Samples of the 2011 and 2016 Surveys

Discussion
The 2016 PPS revealed that 44.5% of patients in Italian acute-care hospitals received 1 or more antimicrobials on the day of the survey. AMU prevalence was almost identical as in 2011, even though there were significant differences in case mix between the 2 surveys. These differences reflect the demographic changes that are occurring in Italian hospitals: patients are increasingly older, with several comorbidities, and they are more frequently subject to invasive devices. However, the Italian AMU prevalence in 2016 was significantly higher than the overall EU/EEA weighted prevalence (30.5%),Reference Plachouras, Kärki and Hansen5 which decreased slightly between 2011 and 2016.4 The European countries participating in the second survey reported an AMU prevalence ranging from 15.9% (Hungary) to 55.6% (Greece), with Italy ranking fifth among the highest antimicrobial consumers.Reference Plachouras, Kärki and Hansen5
Analyzing the indications for antimicrobial prescriptions, several potential targets for improvement were identified. Almost 20% of all antimicrobials were administered for the treatment of hospital-acquired infections, which highlights the need for improved infection prevention and control (IPC) initiatives. Furthermore, a reduction in unnecessary AMU might be achieved by improving surgical prophylaxis prescribing practices. Agents administered for surgical prophylaxis accounted for >1 in 5 of all antimicrobials prescribed on the day of the survey, and in >60% of cases, surgical prophylaxis applications lasted >1 day, contrary to the recommended duration for most surgical procedures.12 Finally, further improvements in prescribing appropriateness are required for medical prophylaxis, which accounted for nearly 25% of all antimicrobials. This percentage is much too high considering that the number of indications for medical prophylaxis are limited3 and that this proportion is more than twice the EU/EEA proportion.Reference Plachouras, Kärki and Hansen5 Furthermore, for >30% of all antimicrobials prescribed for prophylaxis, no indication for the prescription was documented in the patients’ medical records.11
The 2016 survey revealed that BS/LLAs accounted for >60% of all antimicrobials for systemic use, the second-highest proportion among the EU/EEA countries participating in the PPS.Reference Plachouras, Kärki and Hansen5 The extensive use of BS/LLAs reflects a significant selection pressure for the emergence of AMR and constitutes a serious risk for patient safety in a country already facing hyperendemic levels of resistance.13
The most commonly prescribed agent in 2016 was piperacillin plus BLI, which rose significantly compared to the 2011 survey, in line with the EU/EEA trend.14 The growing use of penicillins combined with BLIs may be explained by the increasing severity in case mix. On the other hand, the use of third-generation cephalosporins and fluoroquinolones significantly decreased between the 2 surveys. This decrease is likely due to the high resistance levels of Klebsiella pneumoniae and ESBL-producing microorganisms, although effective stewardship program aimed at reducing healthcare-associated Clostridium difficile infections might also have contributed.
Interestingly, we detected no significant increase in the use of carbapenems, which are generally regarded as the treatment of choice for severe infections by ESBL-producing gram-negative bacteria.Reference Pana and Zaoutis15 This finding may reflect the implementation of carbapenem-sparing strategies. Piperacillin plus BLI was the most common agent used for medical prophylaxis, whereas carbapenems could have been more often reserved for treatment indications, as last-line therapy for serious infections involving multidrug-resistant bacteria.Reference Harris, Peleg and Iredell16
However, carbapenem consumption in Italy remains problematic, and increased stewardship efforts are necessary to reduce the selective pressure for the emergence of resistant bacteria. In this study, we identified stable or decreasing carbapenem resistance levels, although they remain alarmingly high for both K. pneumoniae (50%) and Acinetobacter baumannii (>75%). Furthermore, the percentage of carbapenem-resistant K. pneumoniae found in this study was much higher than the percentage reported by the European Antimicrobial Resistance Surveillance Network (EARS-Net) for Italy in 2017 (29.7%).17 Thus, the situation in acute-care hospitals may be even more severe than that of community-acquired infections. Carbapenem-resistance is a serious public health threat because alternative treatments are limited.Reference Magiorakos, Burns and Baño18 Polymyxins (mainly colistin) are an effective treatment option, but resistance to these agents may develop in treated patients.19 According to ECDC data, polymyxin consumption in Italy is the fourth highest in Europe and is significantly increasing.14 Furthermore, colistin-resistant, carbapenem-resistant, and pan-drug–resistant K. pneumoniae isolates are rapidly disseminating throughout our country.Reference Albiger, Glasner and Struelens20
The hospital indicator data collected in the 2016 PPS highlighted a shortage of staff dedicated to IPC and antimicrobial stewardship: a median of 1.0 full-time equivalent (FTE) IPC nurses (interquartile range, IQR, 0.0–2.0), 0.5 FTE IPC doctors (IQR, 0.0–1.0), and 0.0 FTE antimicrobial stewardship consultants (physicians or pharmacists) (IQR, 0–0.6) per hospital were found. The number of FTE IPC nurses and doctors has decreased by nearly half since 2011.6 To advance the IPC and antimicrobial stewardship interventions that were identified in this study, an increase in dedicated resources is required. Furthermore, considering the significant interregional variations in AMU prevalence found in this study, a more efficient national strategy is needed to improve the lack of standardization and coordination of IPC organization and initiatives between regions.Reference Moro, Marchi and Buttazzi21,22
This study has several limitations. First, the cross-sectional design of the PPS only allowed a direct estimate of AMU prevalence on the day of the survey. Secondly, even though the sample was considered representative, in some regions participation was extremely limited. Given the strong regional disparities in our country, results may not reflect the heterogeneity of Italian health care. Furthermore, the proportions of resistant isolates identified in this study may not be an accurate estimate; for nearly half of all identified HAIs, a positive microbiological test was not available.
Italy continues to be one of the largest consumers of antimicrobials and broad-spectrum antimicrobials in Europe.14 The rates of AMR pathogens in Italy are also among the highest in Europe and are reaching hyperendemic levels.13 Clinical consequences are already apparent: approximately one-third of all deaths in Europe attributable to AMR infections in 2015 were in Italy.Reference Cassini, Högberg and Plachouras2 To address this increasingly severe issue and to define targets for the reduction of HCAIs and antimicrobial consumption, the Italian Ministry of Health issued in 2017 a national action plan to fight antimicrobial resistance.23 The main objectives of the plan are (1) to reinforce and increase the representativeness of AMR surveillance, (2) to implement a national surveillance system for HAIs, (3) to optimize the monitoring of antimicrobial consumption, (4) to promote the adoption across regions of evidence-based IPC practices, (5) to promote an appropriate and conscientious use of AMs, (6) to promote awareness of AMR through the reinforcement of effective communication and information, (7) to promote interdisciplinary training interventions to improve antimicrobial use and IPC practices, and (8) to promote research in the field of AMR, with a specific focus on assessing the effectiveness of surveillance and control interventions. The results of the current study offer scientific support for the objectives of the national action plan and repeated prevalence surveys will facilitate the maintenance and monitoring of the effectiveness of the national strategy.
Acknowledgments
The authors gratefully acknowledge all participating hospitals and would like to thank the regional coordinators: Roberto Novati (Valle d’Aosta), Camilla Sticchi (Liguria), Maurizio Bersani (Lombardia), Ugo Fedeli (Veneto), Luca Fabbri (Provincia Autonoma di Trento), Silvio Brusaferro (Friuli Venezia Giulia), Maria Luisa Moro and Enrico Ricchizzi (Emilia Romagna), Anna Poli (Toscana), Gianni Giovannini (Umbria), Marcello D’Errico (Marche), Vincenzo Puro (Lazio), Giustino Parruti (Abruzzo), Giancarlo Ripabelli (Molise), Bruno Sarnelli (Campania), Rosa Prato (Puglia), Maria Pavia (Calabria), Antonella Agodi (Sicilia), Ida Mura (Sardegna). The authors especially thank the hospital staff involved in data collection for their dedication and willingness to participate. A full list of all participating staff can be found in the Italian 2011 and 2016 HAI PPS reports.6,11
Financial support
This work was supported within the project “Sorveglianza nazionale delle infezioni correlate all’assistenza” (Central action of the CCM, Centro Nazionale per la Prevenzione e il Controllo delle Malattie, 2015).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





